
Electron Transfer & Catalysis in Natural & Synthetic Protein
Biological Electron Transfer:
Living organisms are powered by a stream of electron transfer reactions that pass through a series of proteins imbedded in bioenergetic membranes, such as the mitochondrial membrane shown below. These proteins are designed by blind natural selection to couple electron transfer to transmembrane electrical and pH gradients by placing redox centers of several different types close enough together (usually 14 Å or less) to allow electrons to tunnel through the insulating protein medium from one center to the next on the physiologically acceptable timescale of milliseconds or less.
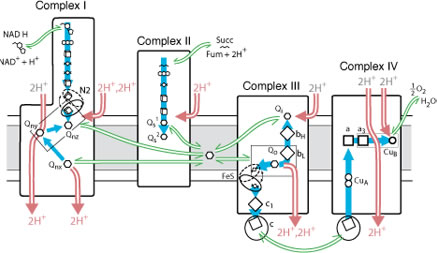
The relatively simple rules of electron tunneling engineering that Nature uses leads to resilient designs that survive the insults of repeated cycles of mutation and natural selection. Click here for a quick calculator of electron tunneling rates between redox cofactors in proteins.
Here we include a link to an applet that demonstrates the fundamental parameters that determine electron transfer.
ET-rates appletSynthetic Proteins-- Maquettes:
Our understanding of these simple rules of electron transfer protein engineering are best tested by building completely artificial electron transfer protein systems, with no sequence similarity to natural proteins. Besides confirming the principles of design, artificial proteins, which we call maquettes, allow the freedom to redesign electron transfer systems in robust packages that can be useful for energy capture and catalysis.
Besides providing an example of the first, completely unnatural oxygen transport protein, our recently published work (Nature, 458, 305-309, 19 March 2009) on the design and engineering of an oxygen transport maquette (akin to human myoglobin and neuroglobin) has allowed us to develop and test the principles of how to build function into artificial proteins while avoiding dead-ends due to protein complexity. Design inhibiting complexity is a common property in natural proteins subject to repeated natural selection. Maquettes intentionally have no sequence similarity to any natural protein and can avoid inheriting this complexity.
When electron tunneling is coupled to bond making and breaking in catalysis, extra energetic barriers must be surmounted that slow down reaction rates. To acheive overall millisecond rates that are fast enough for physiological needs, redox centers in Nature are placed close enough to facilitate inherently fast electron tunneling.
