
This Doctor Found His Own Miracle Drug. Now He Wants to Do It for Others
Dr. Fajgenbaum and his research on Castleman's disease featured in the Wall Street Journal
A transformative mRNA vaccine discovery that's helping prevent COVID-19 around the world.
One Penn Medicine. One Research, drives integrated, innovative research across the Penn Medicine Health System, providing technology advancements and care for our patients and community.
If you are a health care provider in search of Penn Medicine clinical trials that are enrolling patients please view our clinical research opportunities.
For physicians seeking support for Expanded Access Protocols please contact OCR Regulatory.
The central hub for clinical research is the Office of Clinical Research, whose goal is to foster collaborative and innovative research that pursues new ways to care for our patients, accelerates patients’ access to experimental therapy and provides Penn Medicine physicians opportunities to engage in our research mission.

Dr. Fajgenbaum and his research on Castleman's disease featured in the Wall Street Journal

The BRCA1 (BReast CAncer gene 1) and BRCA2 are genes that produce proteins crucial for DNA repair. Women with inactivating mutations in BRCA genes face elevated lifetime risks of recurrent breast and ovarian cancers. Dr. Katherine L. Nathanson of the University of Pennsylvania discusses the complexities of BRCA related breast & ovarian cancers, ongoing research and the potential avenues for future breakthroughs.
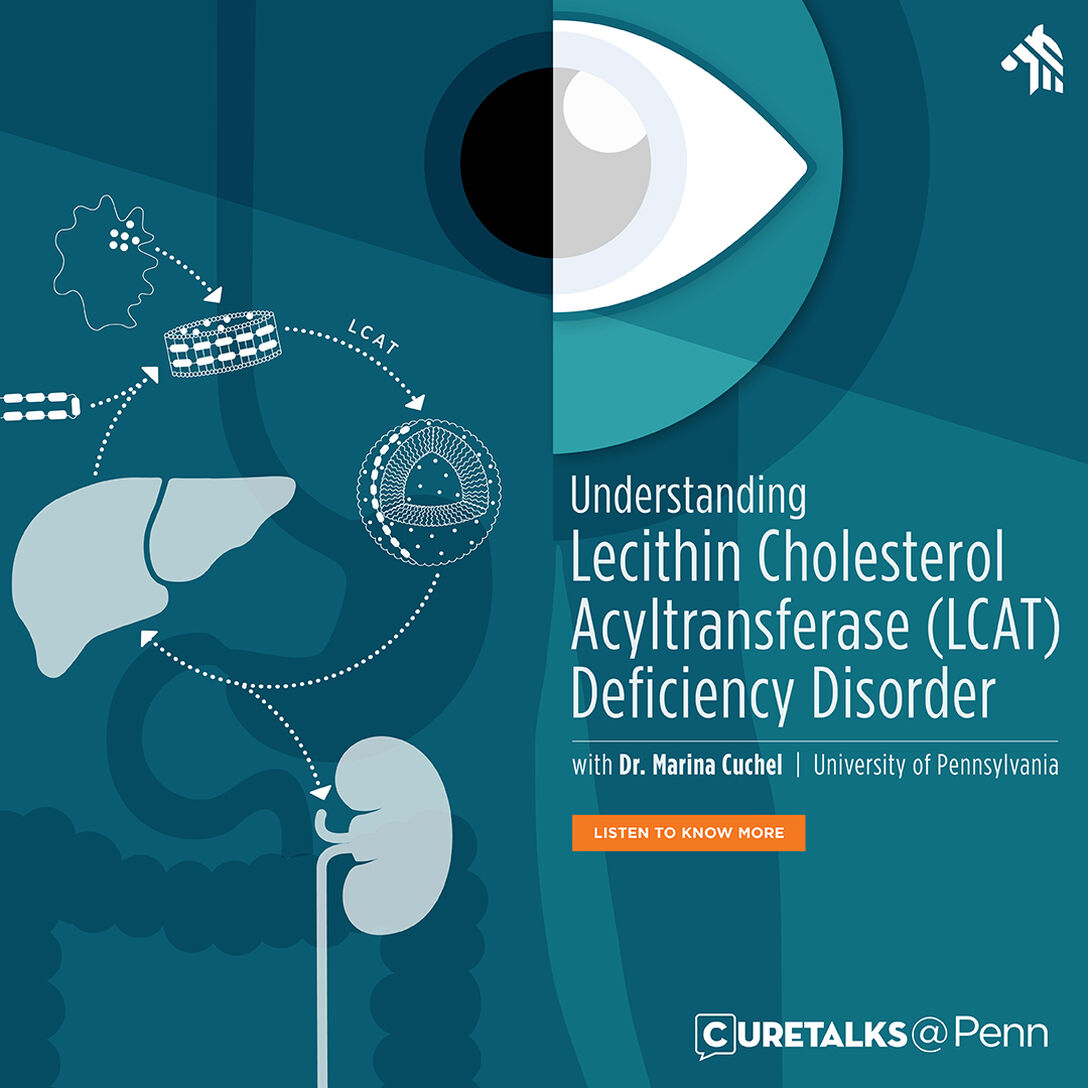
Dr. Marina Cuchel speaks about Lecithin Cholesterol Acyltransferase (LCAT) deficiency, a genetic disorder that affects the body’s ability to process cholesterol. It is characterized by cloudiness of the clear front surface of the eye (corneal opacities), a shortage of red blood cells (hemolytic anemia), and kidney failure.

AACI Clinical Research Innovation 2023 Abstracts and Posters book is now available featuring seven abstracts, posters from ACC-CRU Clinical Research Professionals
News Archive Have a highlight you’d like to see featured here? Submit your highlight idea.