Rompolas Lab
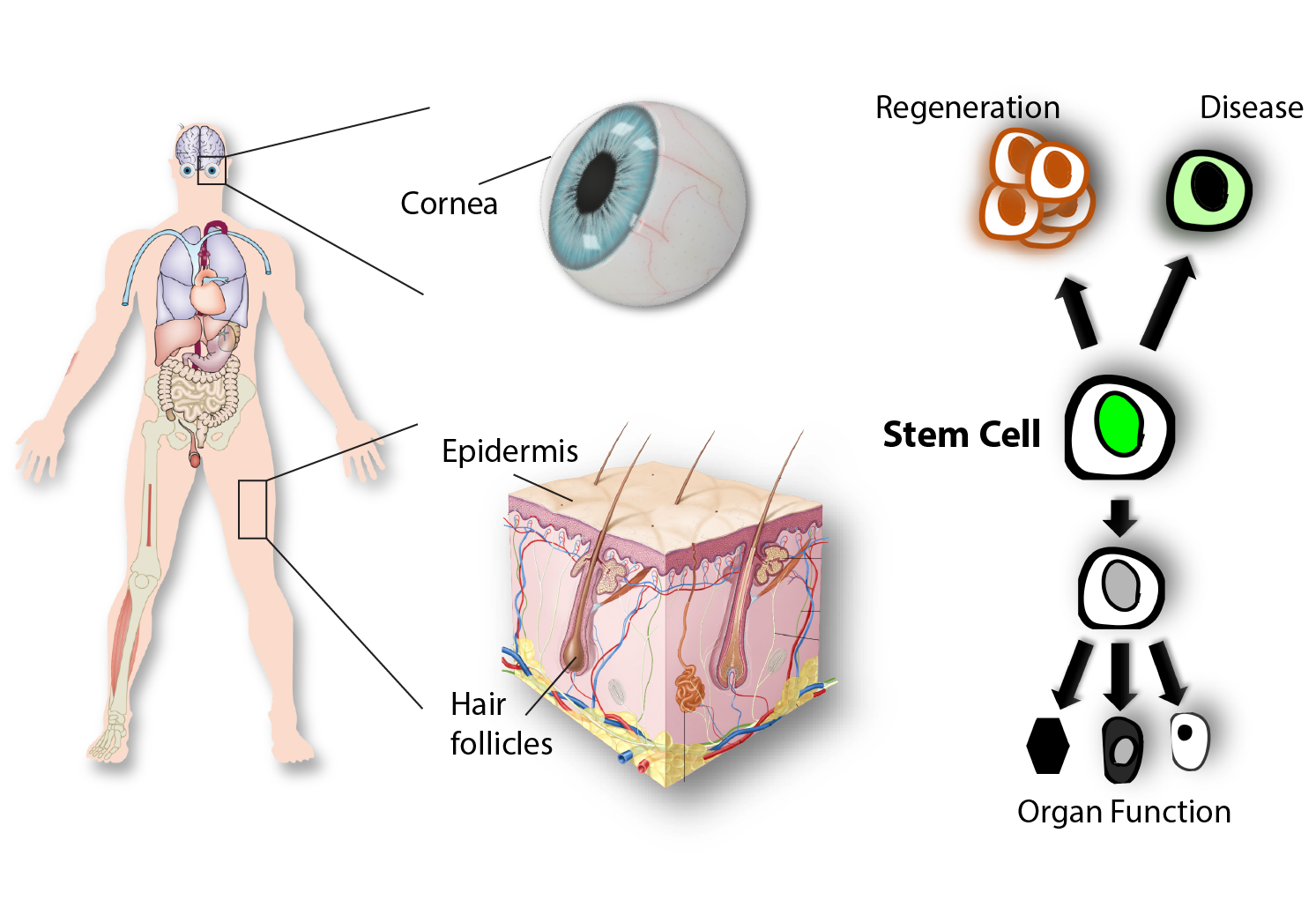
Stem cells in tissue regeneration and disease
Adult stem cells have the unique capacity to generate all the differentiated cell types within the tissue, while also maintaining their own pool throughout life. These properties make stem cells indispensable for the maintenance and regeneration of adult organs. Barrier epithelia in the skin and eye are maintained through a seemingly continuous regeneration process fueled by stem cells dispersed throughout their basal layer. Other epithelial structures, including skin appendages such as hair follicles have distinct niches and stem cells that serve to maintain and regenerate them.
Our research seeks to answer the following fundamental questions: (a) which stem cells support tissue regeneration and how? (b) how the niche microenvironment regulates their activity? (c) how their abnormal behavior contributes to disease pathophysiology?
We are investigating these questions using the mammalian skin and eye as our primary model systems. We aim to leverage our knowledge to develop therapeutic strategies that activate and guide adult, tissue-resident stem cells to optimally support and regenerate organs that fail due to aging or disease.
Live imaging of skin regeneration
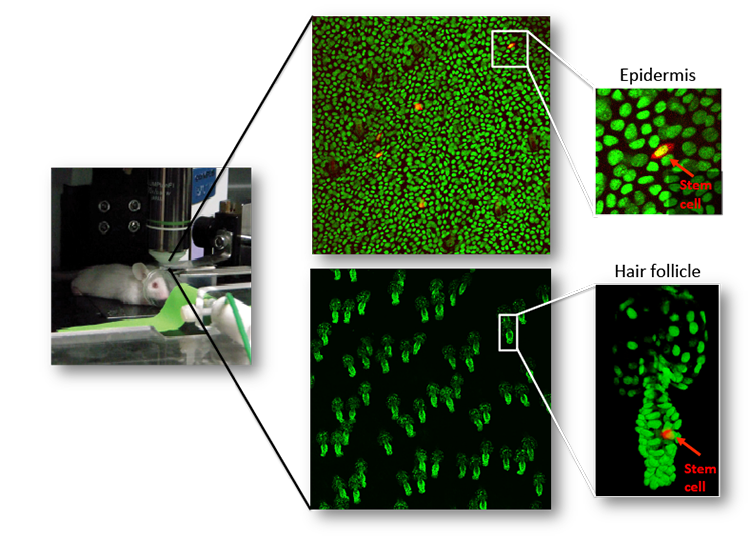
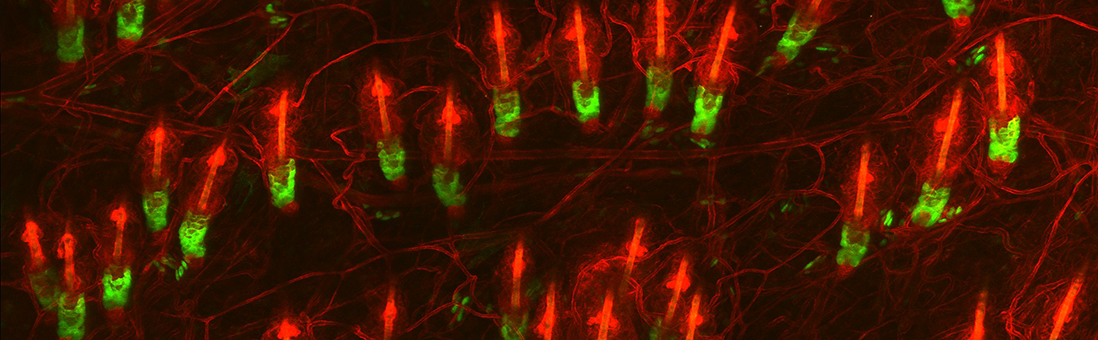
A major effort in our lab is to uncover the organization of the relevant stem cell populations in the skin and to resolve their differentiation trajectory and contribution to tissue homeostasis and regeneration. By pioneering the development of state-of-the-art imaging approaches, we can now directly observe epidermal stem cells in the skin of live mice (Pineda et al. Nat Protoc. 2015; Huang et al. Exp Dermatol 2017). By capturing and analyzing the complete lifecycle of individual stem cells we resolved the mechanism of epidermal maintenance that relies on the spatiotemporal coordination of stem cell behaviors (Rompolas et al. Science 2016).
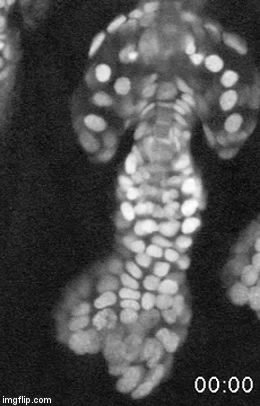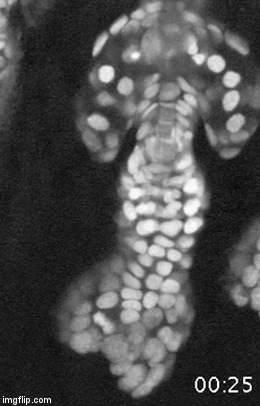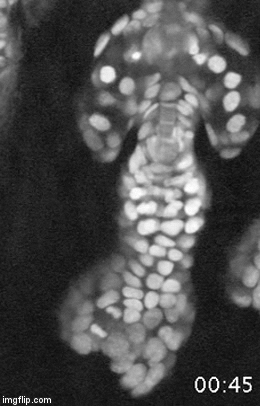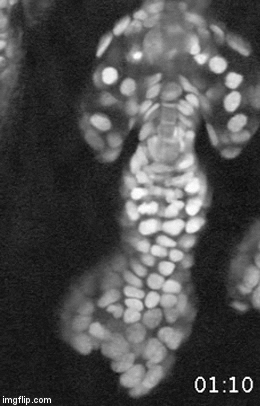
Stem cells rely on cues from their tissue microenvironment to regulate their activity. The skin is a highly innervated tissue but the role of the peripheral nervous system in skin physiology has not been fully resolved. We discovered that in addition to their established sensory function, cutaneous nerves provide a regulatory niche for a unique population of stem cells in the skin epidermis, which specializes in injury response (Huang et al. Cell Stem Cell 2021a).
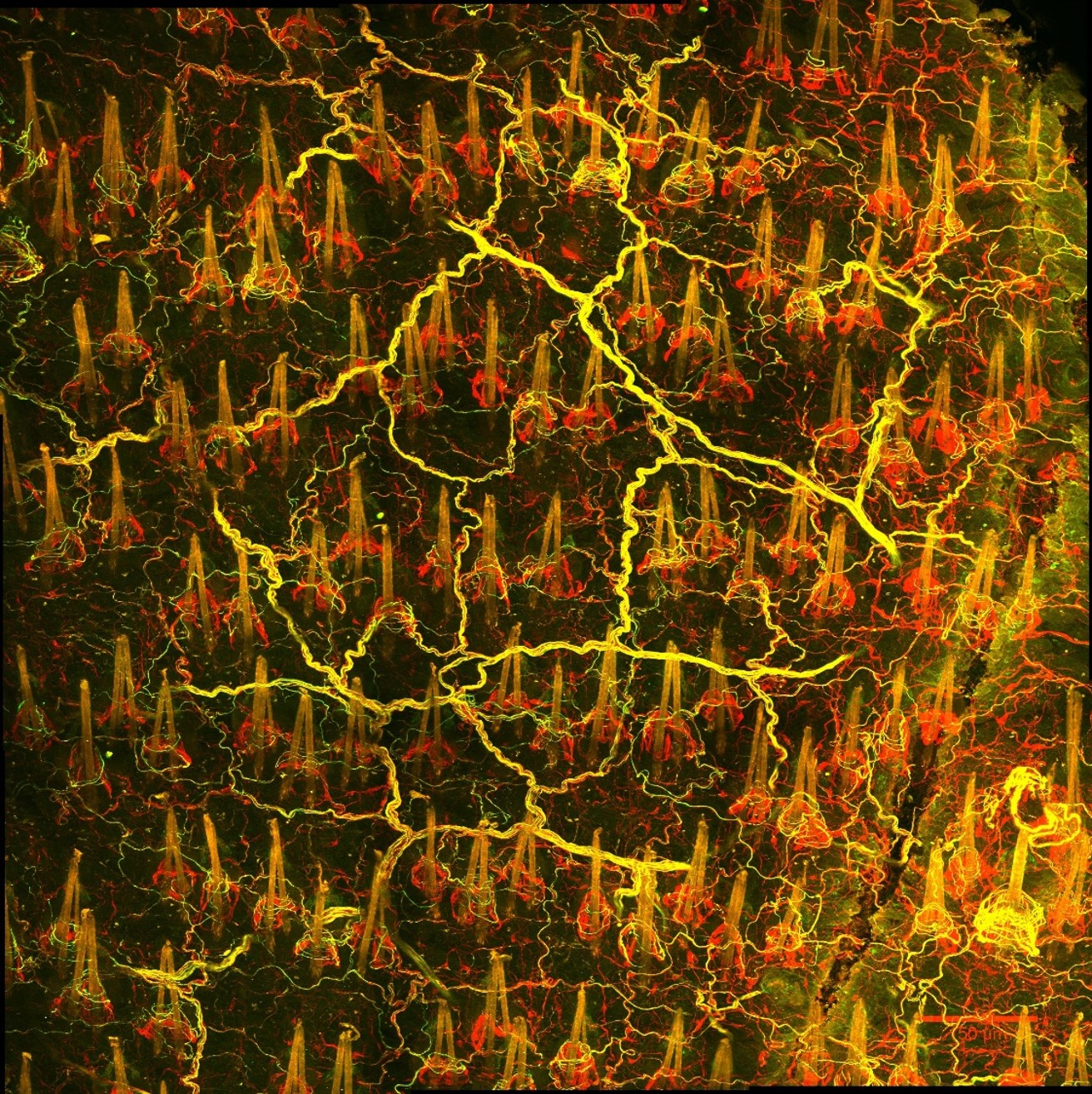
In parallel studies, we asked how epidermal stem cells respond to changes in their physical environment. By applying mechanical stressors to the live mouse skin, we captured previously unknown intracellular “stress vesicles” in epidermal stem cells, which deform their nucleus and trigger their commitment to terminal differentiation. We demonstrated that this mechanism is mediated by calcium signaling through the mechanosensitive ion channel Piezo 1. (Huang et al. Biorxiv 2022).
Ongoing and future directions: To resolve the molecular mechanism of interaction between sensory nerves and stem cells in the skin and ocular surface epithelia and understand how calcium dynamics communicate physical cues to the core transcriptional machinery to control stem cell fate, our lab has established novel in vivo assays, including optogenetics and calcium imaging. Based on our data we propose a generalizable role for the peripheral nervous system as a key regulator of tissue regeneration.
Close
Stem cells in the eye
The cornea is the primary window of our visual system, through which light is refracted and transmitted to the retina. The self-contained tissue organization, optical clarity, immune privilege and pronounced regenerative potential of the cornea presented our lab with a unique opportunity for growth by adopting it as a powerful and clinically relevant model system for basic and translational stem cell research.
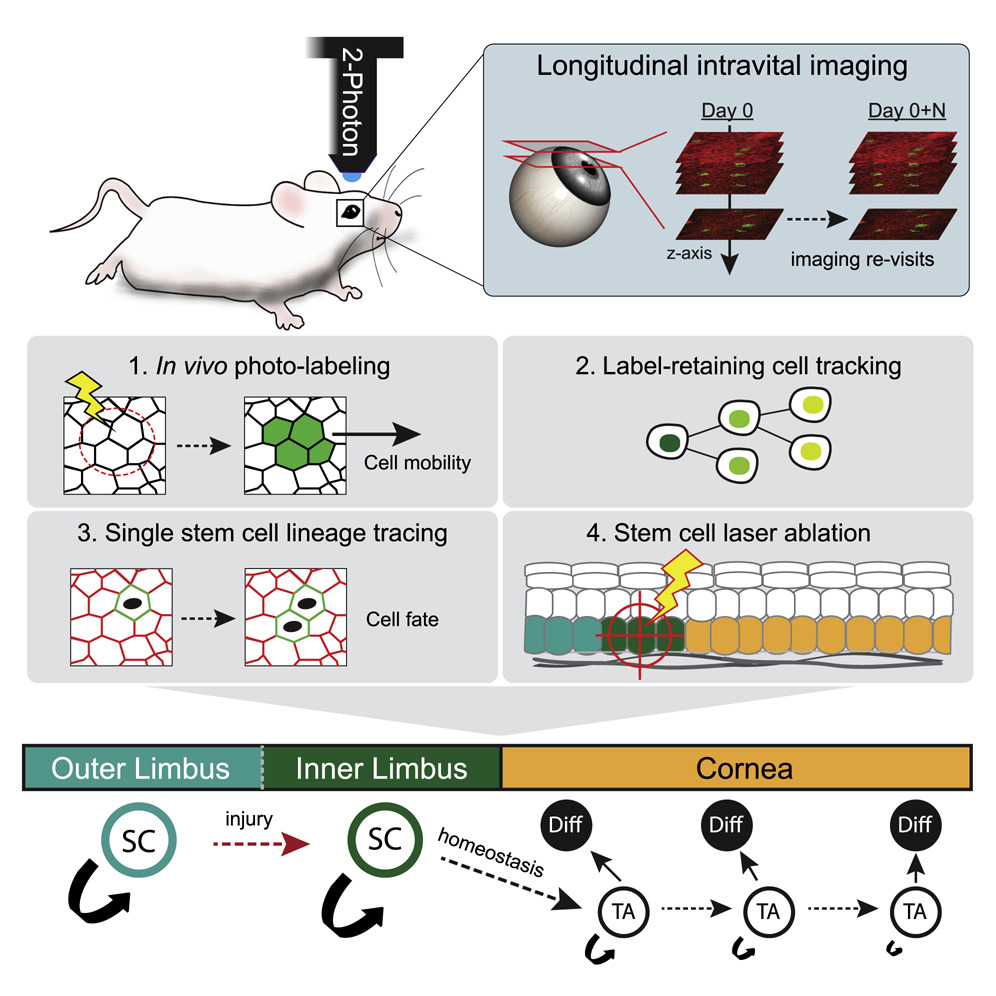
In the past four years we created, from the ground up, mouse models and imaging tools to visualize and track stem cell activity in the intact cornea of live mice by 2-photon microscopy. A major contribution was our discovery of distinct stem cell populations in the corneal stem cell niche. We demonstrated that these stem cells make diverse contributions to tissue maintenance and wound healing (Farrelly et al. Cell Stem Cell 2021b). This paradigm-shifting study demonstrated how the compartmentalized organization of functionally diverse stem cell populations supports the maintenance and regeneration of an adult organ.
Ongoing and future directions: Our current goal is to identify intrinsic and extrinsic regulators of stem cell activity and to test their role and requirement for corneal regeneration. Towards this, we performed single cell genomic analyses and identified several novel markers for corneal stem cells. Using conditional in vivo models, we found that a key transcription factor is a critical for corneal stem cell differentiation. Our long term goal is to resolve the molecular and cellular mechanisms that regulate fate decisions across the ocular surface and to understand their role in disease pathophysiology.
Close
Bench-to-bedside research
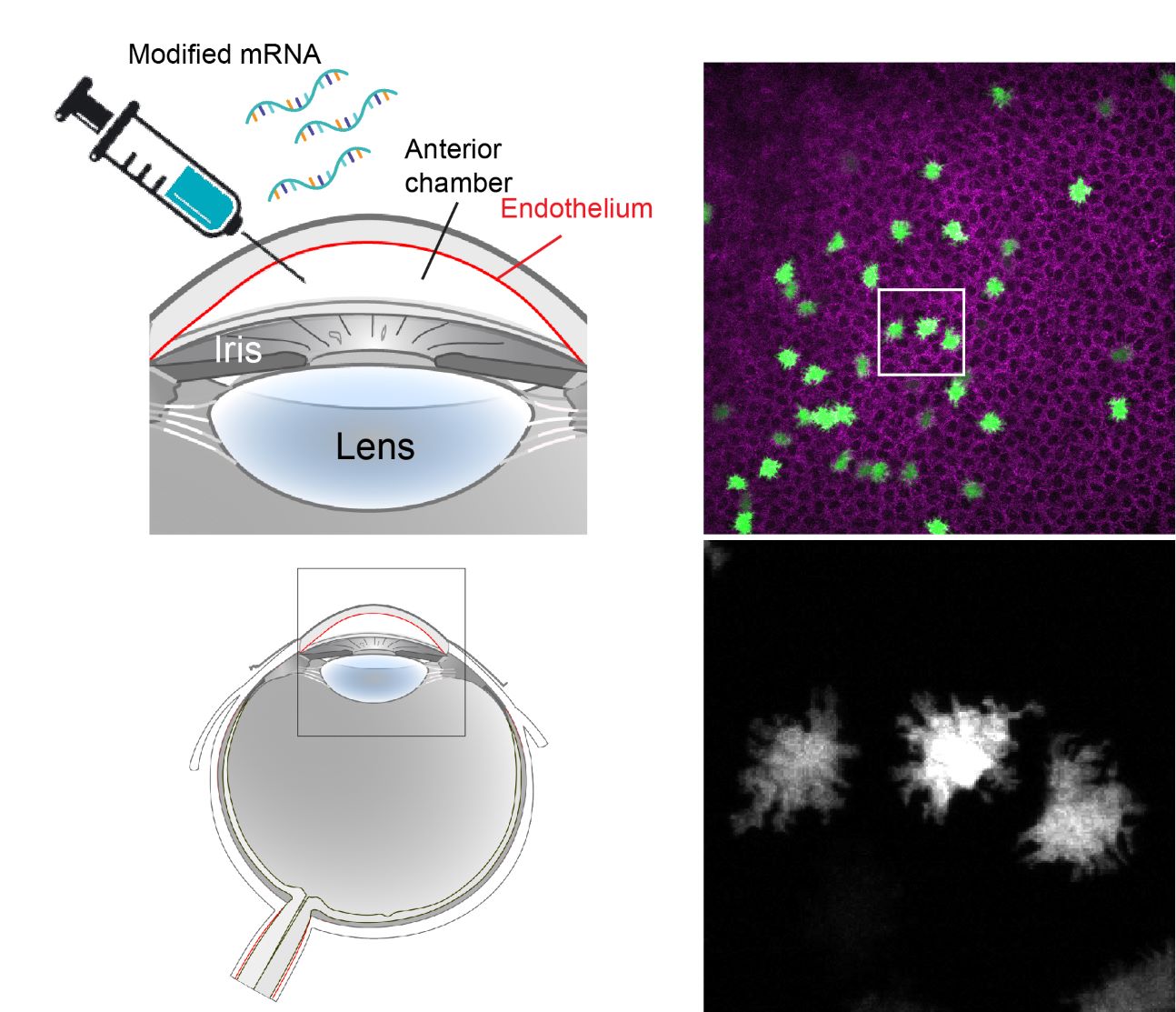
Our newly developed experimental tools for the cornea have enabled parallel studies with a direct translational focus. In contrast to the epithelium, corneal endothelial cells do not regenerate and their decline due to aging or disease leads to loss of vision. To address this unmet clinical need we developed and validated an in vivo model of corneal endothelial dysfunction by high-throughput longitudinal live imaging. Moreover, we established a methodology for induced protein expression using locally delivered modified mRNA. Leveraging these tools, we have generated in vivo proof-of-concept data for a therapeutic approach that aims to enhance regeneration of the corneal endothelium by inducing transient proliferation of quiescent cells, using modified mRNAs encoding for proteins involved in cell cycle activation. This foundational study presents our lab with exciting opportunities for bench-to-bedside research that we intend to vigorously pursue.
Close
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser. | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team.
