What is RVCL? (also known as RVCL-S, CRV, or HERNS)
Retinal vasculopathy with cerebral leukoencephalopathy (RVCL or RVCL-S) is a rare genetic disease that affects the small blood vessels. RVCL is caused by inherited mutations in the TREX1 gene.
RVCL is associated with damage to multiple organs including the brain, eyes, kidneys, liver, and bones. This leads to disability, vision loss, and premature death in all cases, but we are working to find effective treatments and to develop personalized medicines that target TREX1. There are fewer than 200 known patients and 50 families with RVCL in the world.
Click the menus below to learn more about RVCL, including genetics, diagnosis, symptoms, and treatments.
Background and Genetics
What does RVCL stand for?
-
RVCL or RVCL-S stands for Retinal Vasculopathy with Cerebral Leukoencephalopathy (and Systemic manifestations), also known as retinal vasculopathy with cerebral leukodystrophy, cerebroretinal vasculopathy (CRV), or hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS). Other abbreviations also have been used, including CHARIOT and HVR.
-
The name of the disease is explained as follows:
-
Retinal = relating to the retina or the portion of the eye that senses light and sends images to the brain
-
Vasculopathy = blood vessel disease
-
Cerebral = brain
-
Leukoencephalopathy = white matter disease
-
Systemic manifestations = an all-encompassing term reflecting the fact that patients with RVCL also develop kidney, liver, and sometimes also gastrointestinal, thyroid, and bone disease. Thus, RVCL is not just a disease of the brain and the eye. It is truly a multi-system disease.
-
How is RVCL inherited?
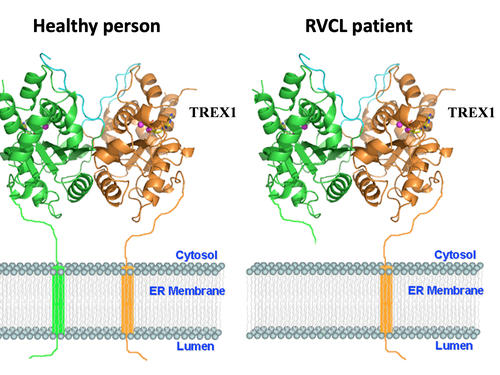
RVCL is caused by mutations in a gene called TREX1, which is found on chromosome 3. RVCL is inherited in a autosomal dominant pattern, which means that about half of family members are affected.
Each affected individual has a 50% chance of passing the disease to each child. Every person has two copies of the TREX1 gene, one copy inherited from each parent. Patients with RVCL have one normal copy of TREX1, and one mutated copy of TREX1. Only one copy of TREX1 is passed to the next generation, which is why there is a 50% chance of passing the disease to each child.
If you would like to find out about a procedure called pre-implantation genetic testing, you can read more about this on the "Finding a Cure" section of our web site. Pre-implantation genetic testing is a procedure that allows couples to perform in vitro fertilization and then choose embryos without the TREX1 mutation for transfer to the uterus. If you have questions, you can contact us directly.
What is the TREX1 gene?
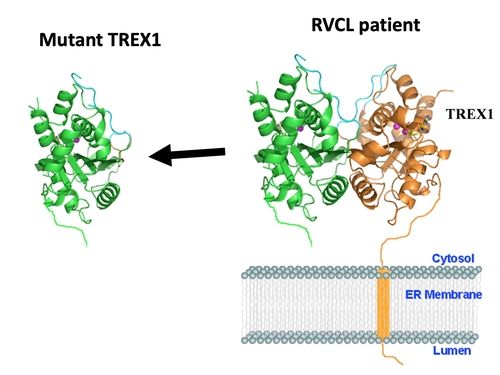
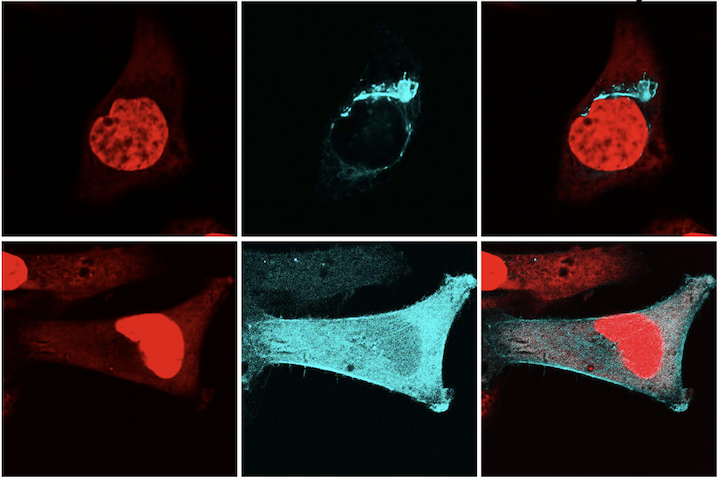
Each gene is the blueprint for a protein, and proteins are the machinery that make the human body work. The TREX1 gene encodes the TREX1 protein. Normally, the TREX1 protein is attached to a membrane inside the cell. In patients with RVCL, one copy of TREX1 is abnormally shortened because of the mutation. This causes the TREX1 protein to float freely in the cell, rather than being anchored to the membrane. Mislocalization of the TREX1 protein somehow causes cellular damage and disease. The exact mechanism remains to be defined, but therapies targeting the mutated TREX1 gene and protein are currently in development.
Symptoms and Diagnosis
Diagnosis
RVCL is diagnosed based on a specific set of TREX1 gene mutations.
The mutations that cause RVCL are in a specific region of the TREX1 gene. Other mutations in TREX1 can cause a other diseases, but all the mutations that cause RVCL are within a specifically defined region.
Unfortunately, many patients with RVCL are not genetically tested, and those patients can end up being diagnosed as having multiple sclerosis, brain tumors, lupus, or vasculitis. Those diseases are much more common than RVCL. Since most physicians have not heard of RVCL, they often presume that patients with RVCL have one of these more common diseases. Genetic testing, which should only be performed under the direct guidance and support of expert physicians and genetic counselors, can be used to accurately diagnose RVCL.
We are hoping to raise awareness of RVCL so that more patients can receive the correct diagnosis. However, before genetic testing, it is important to consult with expert physicians and a genetic counselor with expertise in RVCL. Our team can help with this process.
Symptoms
Early symptoms
Most patients with RVCL are generally healthy until around age 40-50. Occasionally, patients with RVCL develop symptoms earlier or later in life. Early symptoms can include Raynaud’s syndrome (fingers changing color in the cold), blind spots, or small strokes. Many patients develop fatigue, partial loss of vision, or memory problems. Other patients develop kidney, thyroid, or liver abnormalities earlier in the course of disease.
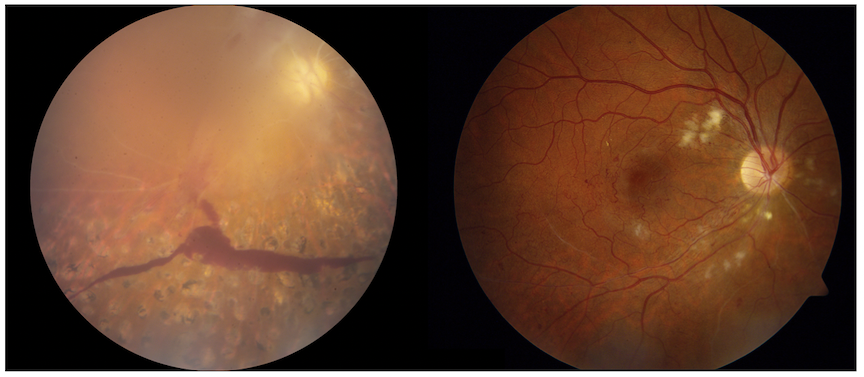
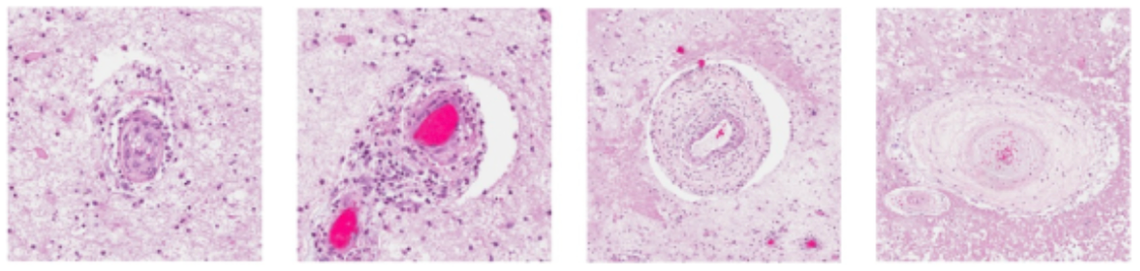
Vasculopathy and organ damage in RVCL
One major process underlying RVCL is related to loss of small blood vessels. (Vasculopathy means “disease of the blood vessels.”) Loss of blood flow leads to progressive organ damage. Examples of organ damage in RVCL include chronic kidney disease, loss of blood flow to the retina leading to blindness or glaucoma, and strokes leading to neurological symptoms. Sometimes new symptoms can arise suddenly (e.g., glaucoma or strokes), but usually the disease is slowly progressive over the course of many years.
Impact of RVCL on the brain
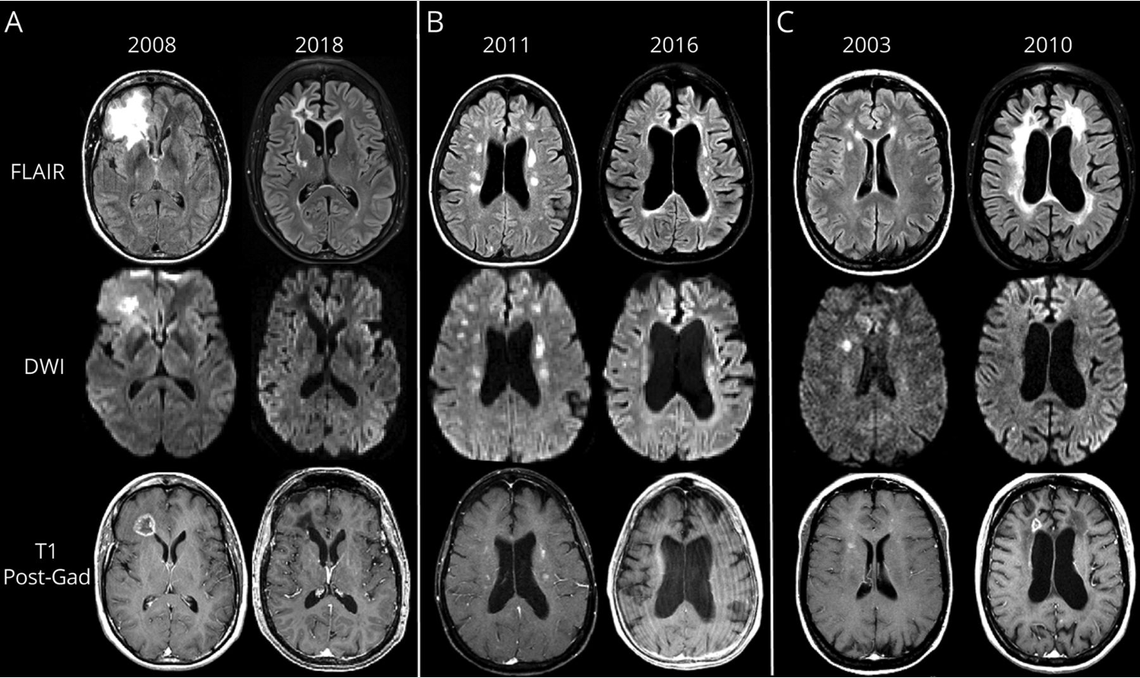


 Treatment
Treatment Learn more by exploring these links:
Learn more by exploring these links: