Center for Hereditary Retinal Degenerations
Our Center aims to provide better sight for people living with inherited retinal diseases (IRDs) such as retinitis pigmentosa, Stargardt disease, Leber congenital amaurosis, blue cone monochromacy, choroideremia and many others. Co-director Dr. Aleman provides clinical care to IRD patients who carry the burden of progressive vision loss that is currently not treatable. Co-directors Dr. Cideciyan and Dr. Aleman perform world-class research in clinical trials and in the laboratory to make progress towards treatments.
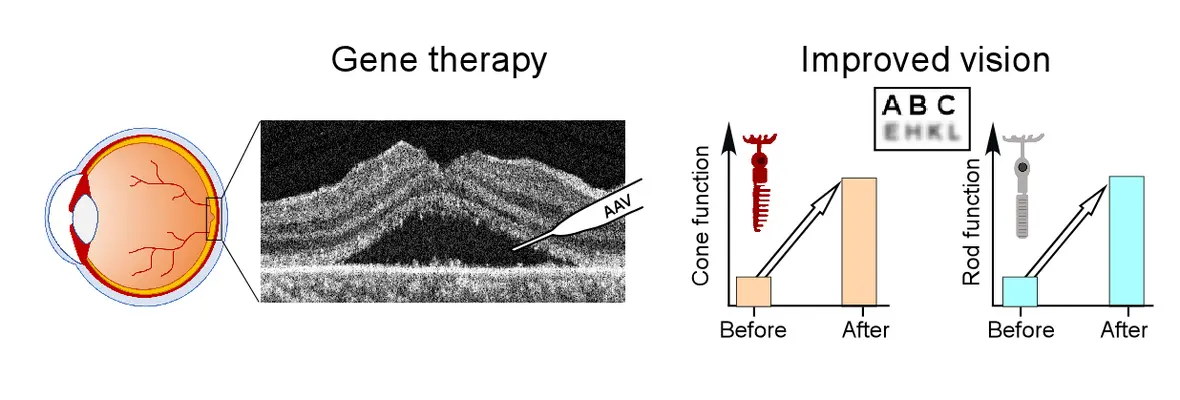
September 2024: Gene therapy trial results published in The Lancet
A Phase I/II clinical trial involving subretinal gene therapy for patients with LCA1 caused by GUCY2D mutations has been ongoing at our center since November 2019. 12-month safety and efficacy data from 15 patients enrolled in this trial was published in The Lancet. This investigational treatment is well tolerated and results in large improvements in night vision.
Yang P, Pardon LP, Ho AC, Lauer AK, Yoon D, Boye SE, Boye SL, Roman AJ, Wu V, Garafalo AV, Sumaroka A, Swider M, Viarbitskaya I, Aleman TS, Pennesi ME, Kay CN, Fujita KP, Cideciyan AV. Safety and efficacy of ATSN-101 in patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D: a phase 1/2, multicentre, open-label, unilateral dose escalation study. Lancet 404:962-970, 2024. [DOI] [UPenn Press Release] [Atsena Press Release] [Clinicaltrials.gov] [Comment]