Center for Hereditary Retinal Degenerations
Our Center aims to provide better sight for people living with inherited retinal diseases (IRDs) such as retinitis pigmentosa, Stargardt disease, Leber congenital amaurosis, blue cone monochromacy, choroideremia and many others. Co-director Dr. Aleman provides clinical care to IRD patients who carry the burden of progressive vision loss that is currently not treatable. Co-directors Dr. Cideciyan and Dr. Aleman perform world-class research in clinical trials and in the laboratory to make progress towards treatments.
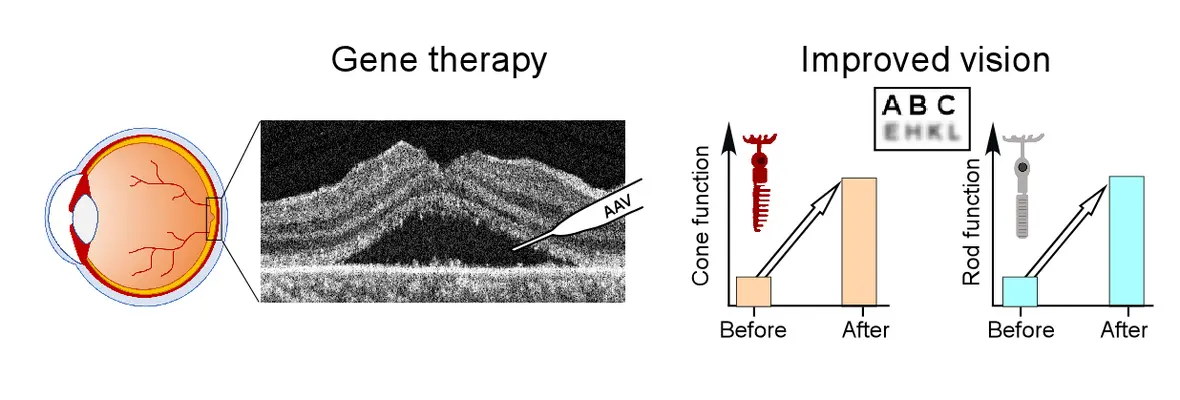
July 2025: LCA5 gene therapy trial results published in Molecular Therapy
Two years ago we started a phase 1/2 clinical trial of gene augmentation therapy in LCA5 - a severe early-onset retinal disease. Recently preliminary results from this trial was published. Efficacy was detectable by subjective and objective methods at 1-month post-treatment and persisted for at least 12 months. Improvements in cone-mediated day-vision were detected perceptually and by pupillometry. Following gene therapy there was better performance on a virtual reality orientation and mobility test. Visual acuity returned to baseline or improved in the treated eyes of all participants.
Aleman TS, Uyhazi KE, Roman AJ, Weber ML, O'Neil EC, Swider M, Sumaroka A, Maguire KH, Aleman EM, Santos AJ, Kim RJ, Parchinski KM, Billek A, Fradin M, Chung W, Margaritis P, Sun J, Scoles DH, Wu V, Garafalo AV, Jayagopal A, Yerxa B, Tuller S, Maguire AM, Bennett J, Cideciyan AV. Recovery of cone-mediated vision in Lebercilin associated retinal ciliopathy after gene therapy: One-year results of a phase I/II trial. Mol Ther. 2025:S1525-0016(25)00486-1. [DOI] [PubMed]