- Home
- Digital Formulary
Digital Formulary
A digital formulary is a clinically-based collection of digital health solutions, containing connected devices and platforms that clinicians have found useful in day-to-day practice, education, and interactions with patients and colleagues.
How was this formulary populated?
This cardiology-focused digital formulary has been crowdsourced from clinicians, researchers, patients, and health administrators to identify best practices. This is a dynamic process with continuous critique of existing tools and ongoing research for new technologies that may help with care delivery. Crowdsourced entries are then vetted where possible with data from research studies, clinical experiences, and regulatory guidance, and curated into an accessible and standardized collection.
A major consideration for entry inclusion is ease of use and availability of clinical data with minimal clinician effort. Where possible, efforts have been made to develop resources to support use of the device or platform with both technical infrastructure and human technical support.
If there are digital tools that should be on this list, or if there are clinical problems that need to be addressed with this formulary, please let us know.
How should I use this formulary?
We suggest skimming through the formulary for available devices and considering testing any that may be relevant to your practice. Please send any feedback, good or bad, our way.
Instructions on populating the formulary:
Please complete this form to the best of your ability for the digital cardiology solution of interest. Include notes, links, or any other information that you find relevant. We will format and revise as needed.
Hypertension

A&D Medical ULTRACONNECT Wireless Blood Pressure Monitor (UA-1200BLE)
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
A&D Medical ULTRACONNECT Wireless Blood Pressure Monitor (UA-1200BLE)
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Technical Aspects/Integration
Syncs to A&D Medical app on iOS or Android devices via Bluetooth.
Procurement/Cost
$130.24 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020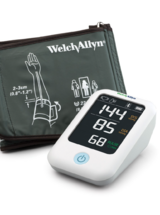
Hillrom-Welch Allyn: Welch Allyn Home®️ Blood Pressure Monitor, 1700 Series (H-BP100SBP)
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Hillrom-Welch Allyn: Welch Allyn Home®️ Blood Pressure Monitor, 1700 Series (H-BP100SBP)
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Technical Aspects/Integration
Syncs to Hillrom-Welch Allyn app on iOS or Android devices via Bluetooth.
Procurement/Cost
$119.99 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020
Omron 5 Series®️ Wireless (BP7250, HEM-7311) Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Omron 5 Series®️ Wireless (BP7250, HEM-7311) Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Models
Technical Aspects/Integration
Syncs to OMRON Connect app on iOS or Android devices via Bluetooth. List of compatible devices here.
Procurement/Cost
$52.49-69.99 (can be purchased via vendor site or Amazon).
Clinical Lead
Notes
Entry Author and Date
Allison Hare, November 2020
Omron 7 Series®️ Wireless (BP7350, HEM-7320) Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Omron 7 Series®️ Wireless (BP7350, HEM-7320) Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Technical Aspects/Integration
Syncs to OMRON Connect app on iOS or Android devices via Bluetooth. List of compatible devices here.
Procurement/Cost
$67.49-89.99 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020
Omron 10 Series®️ Wireless (BP7450, HEM-7320) Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Omron 10 Series®️ Wireless (BP7450, HEM-7320) Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Technical Aspects/Integration
Syncs to OMRON Connect app on iOS or Android devices via Bluetooth. List of compatible devices here.
Procurement/Cost
$74.99-99.99 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020
Omron Complete™ Wireless (BP7900, HEM-7311) Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Omron Complete™ Wireless (BP7900, HEM-7311) Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Technical Aspects/Integration
Syncs to OMRON Connect app on iOS or Android devices via Bluetooth. List of compatible devices here. Can also take an ECG.
Procurement/Cost
$179.95-199.99 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020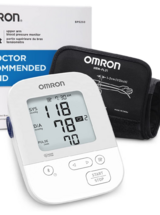
Omron Silver Wireless (BP5250, HEM-7320) Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Omron Silver Wireless (BP5250, HEM-7320) Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
The American Medical Association (AMA) enlisted the National Opinion Research Center at the University of Chicago to assist in the design and management of an independent process to determine which BP devices with active FDA 510(k) pre-market clearance record and documentation in the US meet the AMA’s established criteria to validate clinical accuracy (the “Validated Device Listing”, or VDL Criteria). This process entailed an independent review committee comprised of physician experts assessing whether a BP device meets these VDL Criteria, then processing results into a formal list of BP devices that have been validated for clinical accuracy. This device is on the VDL list.
Technical Aspects/Integration
Syncs to OMRON Connect app on iOS or Android devices via Bluetooth. List of compatible devices here.
Procurement/Cost
$53.68 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020
QardioArm Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
QardioArm Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
FDA-cleared.
Technical Aspects/Integration
Syncs to app on iOS, Android, and Kindle via Bluetooth.
Procurement/Cost
$79.20-99.99 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020
Withings BPM Connect Connected Blood Pressure Cuff
Full descriptionHypertension Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
For monitoring of blood pressure to diagnose and manage hypertension.
Withings BPM Connect Connected Blood Pressure Cuff
Hypertension Obstructive Sleep Apnea Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
For monitoring of blood pressure to diagnose and manage hypertension.
Clinical Indication
- Facilitating the diagnosis of hypertension in alleviating the need for the patient to return to the office for follow-up BP checks
- Increasing accuracy of the diagnosis of hypertension, as ambulatory BP monitoring can expose white coat hypertension (elevated BP in office but not at home) or masked hypertension (elevated BP at home but not in office)
- Monitoring of treatment efficacy to titrate levels of antihypertensive medication
- Improving patient compliance with antihypertensive medication
Evidence Base
- Home blood pressure monitoring more accurately reflects the risk of cardiovascular events than office blood pressure measurement. In addition, there is high-quality evidence that HBPM combined with clinical support improves blood pressure control. Therefore, HBPM is increasingly recommended by guidelines to confirm the diagnosis of hypertension and evaluate the efficacy of blood pressure-lowering medications. Liyanage-Don N, Fung D, Phillips E, Kronish IM. Implementing Home Blood Pressure Monitoring into Clinical Practice. Curr Hypertens Rep. 2019;21(2):14.
- Over the past several decades, evidence has accumulated on the following 2 approaches for measuring blood pressure outside of the clinic: ambulatory blood pressure monitoring and home blood pressure monitoring. Both of these methods have a stronger association with cardiovascular disease outcomes than clinic blood pressure measurement. Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691-700.
- Home blood pressure monitoring is more effective in reducing systolic and diastolic blood pressure compared to other interventions but the clinical significance of its effect is moderate. Re LG, Fusetti V. Efficacia del monitoraggio domiciliare della pressione arteriosa. Sinossi di revisioni sistematiche [Effectiveness of blood pressure home monitoring. Synopsis of systematic reviews.]. Prof Inferm. 2017;70(1):3-11.
Quality Control
FDA-cleared.
Technical Aspects/Integration
Can also measure heart rate. Syncs via Wi-Fi or Bluetooth; results can be sent to provider via iOS app.
Procurement/Cost
$99.95 (can be purchased via vendor site or Amazon).
Entry Author and Date
Allison Hare, November 2020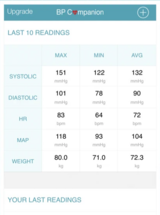
Blood Pressure Companion Hypertension Management App
Full descriptionHypertension Hypertension Mobile applications EHR-adjacent clinician portal Smartphone Smart watch
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Blood Pressure Companion Hypertension Management App
Hypertension Mobile applications EHR-adjacent clinician portal Smartphone Smart watch
Description
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Evidence Base
A systematic review on the effectiveness of apps in lowering blood pressure, as well as their usability and patients' satisfaction with their use, found that most studies reported that apps might be effective in lowering blood pressure and are accepted by users. However, these findings should be interpreted with caution, as most of the studies had a high risk of bias. Alessa T, Abdi S, Hawley MS, de Witte L. Mobile Apps to Support the Self-Management of Hypertension: Systematic Review of Effectiveness, Usability, and User Satisfaction. JMIR Mhealth Uhealth. 2018 Jul 23;6(7):e10723.
Quality Control
Hypertension management apps are not classified as medical devices under Section 201(h) of the FFDCA and are therefore not subject to FDA regulatory oversight.
Technical Aspects/Integration
Can share information with providers via email. Syncs with Apple HealthKit.
Procurement/Cost
Can be downloaded from the Google Play Store or iOS App Store.
Notes
Manually tracks blood pressure, heart rate, and weight data with reminders to take blood pressure readings. Features reminders to take blood pressures and analysis view with 1 day, 1 week, 1 month and 1 year trends.
Entry Author and Date
Allison Hare, January 2021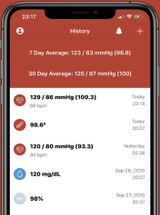
Blood Pressure Tracker+ Hypertension Management App
Full descriptionHypertension Hypertension Mobile applications EHR-adjacent clinician portal Smartphone Smart watch
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Blood Pressure Tracker+ Hypertension Management App
Hypertension Mobile applications EHR-adjacent clinician portal Smartphone Smart watch
Description
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Evidence Base
A systematic review on the effectiveness of apps in lowering blood pressure, as well as their usability and patients' satisfaction with their use, found that most studies reported that apps might be effective in lowering blood pressure and are accepted by users. However, these findings should be interpreted with caution, as most of the studies had a high risk of bias. Alessa T, Abdi S, Hawley MS, de Witte L. Mobile Apps to Support the Self-Management of Hypertension: Systematic Review of Effectiveness, Usability, and User Satisfaction. JMIR Mhealth Uhealth. 2018 Jul 23;6(7):e10723.
Quality Control
Hypertension management apps are not classified as medical devices under Section 201(h) of the FFDCA and are therefore not subject to FDA regulatory oversight.
Technical Aspects/Integration
Syncs with Apple HealthKit.
Procurement/Cost
Can be downloaded from the Google Play Store or iOS App Store.
Notes
Manually tracks blood pressure, heart rate, blood glucose, SpO2, weight, and medication data with analysis view with 1 week, 2 weeks, 1 month, 3 months, 6 months, and 1 year trends.
Entry Author and Date
Allison Hare, January 2021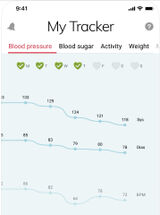
Hello Heart Premium Hypertension Management App
Full descriptionHypertension Hypertension Mobile applications EHR-adjacent clinician portal Smartphone
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Hello Heart Premium Hypertension Management App
Hypertension Mobile applications EHR-adjacent clinician portal Smartphone
Description
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Evidence Base
A systematic review on the effectiveness of apps in lowering blood pressure, as well as their usability and patients' satisfaction with their use, found that most studies reported that apps might be effective in lowering blood pressure and are accepted by users. However, these findings should be interpreted with caution, as most of the studies had a high risk of bias. Alessa T, Abdi S, Hawley MS, de Witte L. Mobile Apps to Support the Self-Management of Hypertension: Systematic Review of Effectiveness, Usability, and User Satisfaction. JMIR Mhealth Uhealth. 2018 Jul 23;6(7):e10723.
Quality Control
Hypertension management apps are not classified as medical devices under Section 201(h) of the FFDCA and are therefore not subject to FDA regulatory oversight
Technical Aspects/Integration
Syncs with Apple HealthKit.
Procurement/Cost
Can be downloaded from the Google Play Store or iOS App Store.
Notes
Manually tracks blood pressure, blood glucose, and weight data with weekly “reports” and daily “insights”, as well as reminders to take blood pressure readings and medications.
Entry Author and Date
Allison Hare, January 2021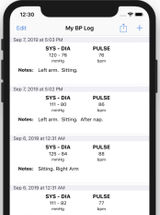
My Blood Pressure Journal Hypertension Management App
Full descriptionHypertension Hypertension Mobile applications EHR-adjacent clinician portal Smartphone
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
My Blood Pressure Journal Hypertension Management App
Hypertension Mobile applications EHR-adjacent clinician portal Smartphone
Description
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Evidence Base
A systematic review on the effectiveness of apps in lowering blood pressure, as well as their usability and patients' satisfaction with their use, found that most studies reported that apps might be effective in lowering blood pressure and are accepted by users. However, these findings should be interpreted with caution, as most of the studies had a high risk of bias. Alessa T, Abdi S, Hawley MS, de Witte L. Mobile Apps to Support the Self-Management of Hypertension: Systematic Review of Effectiveness, Usability, and User Satisfaction. JMIR Mhealth Uhealth. 2018 Jul 23;6(7):e10723.
Quality Control
Hypertension management apps are not classified as medical devices under Section 201(h) of the FFDCA and are therefore not subject to FDA regulatory oversight.
Technical Aspects/Integration
Manually tracks blood pressure and heart rate data with options to share via SMS, email, Facebook or Twitter. Syncs with Apple HealthKit.
Procurement/Cost
Can be downloaded from the Google Play Store or iOS App Store.
Entry Author and Date
Allison Hare, January 2021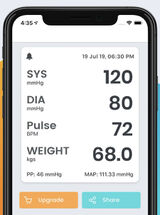
SmartBP Hypertension Management App
Full descriptionHypertension Hypertension Mobile applications EHR-adjacent clinician portal Smartphone Smart watch
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
SmartBP Hypertension Management App
Hypertension Mobile applications EHR-adjacent clinician portal Smartphone Smart watch
Description
App designed to assist patients with tracking, analyzing, and sharing blood pressure measurements.
Evidence Base
A systematic review on the effectiveness of apps in lowering blood pressure, as well as their usability and patients' satisfaction with their use, found that most studies reported that apps might be effective in lowering blood pressure and are accepted by users. However, these findings should be interpreted with caution, as most of the studies had a high risk of bias. Alessa T, Abdi S, Hawley MS, de Witte L. Mobile Apps to Support the Self-Management of Hypertension: Systematic Review of Effectiveness, Usability, and User Satisfaction. JMIR Mhealth Uhealth. 2018 Jul 23;6(7):e10723.
Quality Control
Hypertension management apps are not classified as medical devices under Section 201(h) of the FFDCA and are therefore not subject to FDA regulatory oversight.
Technical Aspects/Integration
Tracks blood pressure and heart rate data via Bluetooth-enabled blood pressure monitors (A&D, Omron, QardioArm, iHealth, and Withings). Syncs with Apple HealthKit. Can share information with providers via email or SMS.
Procurement/Cost
Can be downloaded from the Google Play Store or iOS App Store.
Notes
Also tracks weight and ECG information. Features tagging system for notes about blood pressure readings and analysis view with 1 week, 2 week, and 1 month trends.
Entry Author and Date
Allison Hare, January 2021Heart failure

CardioMEMS Pulmonary Artery Sensor
Full descriptionHeart failure Heart Failure Biosensors EHR-adjacent clinician portal Yes
Implantable sensors that track pulmonary artery pressures to monitor heart failure exacerbations.
CardioMEMS Pulmonary Artery Sensor
Heart Failure Biosensors EHR-adjacent clinician portal Yes
Description
Implantable sensors that track pulmonary artery pressures to monitor heart failure exacerbations.
Clinical Indication
NYHA class III heart failure + admission for decompensated heart failure in past 12 months.
Evidence Base
In 6 months, 84 heart-failure-related hospitalizations were reported in the treatment group (n=270) compared with 120 in the control group (n=280; rate 0·32 vs 0·44, hazard ratio [HR] 0·72, 95% CI 0·60–0·85, p=0·0002). During the entire follow-up (mean 15 months [SD 7]), the treatment group had a 37% reduction in heart-failure-related hospitalisation compared with the control group (158 vs 254, HR 0·63, 95% CI 0·52–0·77; p<0·0001). Our results are consistent with, and extend, previous findings by definitively showing a significant and large reduction in hospitalisation for patients with NYHA class III heart failure who were managed with a wireless implantable haemodynamic monitoring system. The addition of information about pulmonary artery pressure to clinical signs and symptoms allows for improved heart failure management. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658– 666.
Quality Control
FDA-approved for above indication.
Models
Technical Aspects/Integration
No Bluetooth. Data is wirelessly transmitted to home monitoring device and for CardioMEMS, uploaded to Abbott platform via cellular network.
Procurement/Cost
Covered by Medicare if enrolled in Penn CardioMEMS Registry; private payers require prior authorization.
Clinical Lead
Monique Tanna
Notes
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021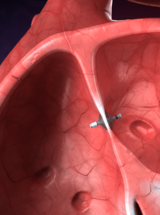
V-LAP Atrial Pressure Monitor
Full descriptionHeart failure Heart Failure Biosensors EHR-adjacent clinician portal
Interatrial septum monitor that provides left atrial pressure readings and physiological data.
V-LAP Atrial Pressure Monitor
Heart Failure Biosensors EHR-adjacent clinician portal
Description
Interatrial septum monitor that provides left atrial pressure readings and physiological data.
Clinical Indication
NYHA class III heart failure.
Evidence Base
Forthcoming - VECTOR-HF clinical trial underway.
Quality Control
FDA Breakthrough Designation.
Technical Aspects/Integration
-
V-LAP device is inserted via a standard, minimally-invasive catheterization procedure
-
Patients put on a light belt that induces power to the implant, wirelessly syncs with it and transmits its monitored data automatically to a cloud-based system. The medical team is then able to access the data, analyze the readings and use the information
Procurement/Cost
Not yet commercially available.
Entry Author and Date
Jonathan Wakim, December 2020
A&D PLUSCONNECT Wireless Weight Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights.
A&D PLUSCONNECT Wireless Weight Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
- Remotely managed patients recently hospitalized for heart failure had fewer unplanned cardiovascular hospitalizations and days in the hospital, lower rates of all-cause and cardiovascular mortality, and improved quality of life compared to patients with usual care only. Prescher S, Koehler K, Koehler F. e-Health in cardiology: remote patient management of heart failure patients. e-Journal of Cardiology Practice. 2020;18(26).
- The randomized, multicenter “Telemedical Interventional Management in Heart Failure II (TIM-HF2)” trial showed for the first time a significant improvement in all-cause mortality based on non-invasive remote management. Telemedicine patients had to spend fewer days in the hospital due to unplanned cardiovascular admissions - on average, 3.8 days compared to 5.6 days in the usual care group. In terms of all-cause mortality, out of 100 heart failure patients in the year of care, only eight patients of the remote management group (7.8 per 100 patient-years) died, while eleven patients died under the usual medical care conditions (11.3 per 100 patient-years). Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-57.
Quality Control
Has not been evaluated by the FDA.
Models
Technical Aspects/Integration
Bluetooth-enabled with vendor app. Weight capacity 400 lbs.
Procurement/Cost
$39.99; can be purchased from Amazon or vendor site.
Clinical Lead
Monique Tanna
Notes
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
A&D Premium Wireless Weight Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights.
A&D Premium Wireless Weight Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
- Remotely managed patients recently hospitalized for heart failure had fewer unplanned cardiovascular hospitalizations and days in the hospital, lower rates of all-cause and cardiovascular mortality, and improved quality of life compared to patients with usual care only. Prescher S, Koehler K, Koehler F. e-Health in cardiology: remote patient management of heart failure patients. e-Journal of Cardiology Practice. 2020;18(26).
- The randomized, multicenter “Telemedical Interventional Management in Heart Failure II (TIM-HF2)” trial showed for the first time a significant improvement in all-cause mortality based on non-invasive remote management. Telemedicine patients had to spend fewer days in the hospital due to unplanned cardiovascular admissions - on average, 3.8 days compared to 5.6 days in the usual care group. In terms of all-cause mortality, out of 100 heart failure patients in the year of care, only eight patients of the remote management group (7.8 per 100 patient-years) died, while eleven patients died under the usual medical care conditions (11.3 per 100 patient-years). Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-57.
Quality Control
Has not been evaluated by the FDA.
Technical Aspects/Integration
Bluetooth-enabled with vendor app. Weight capacity 450 lbs.
Procurement/Cost
$89.99; can be purchased from Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Fitbit Aria Air Digital Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights.
Fitbit Aria Air Digital Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
- Remotely managed patients recently hospitalized for heart failure had fewer unplanned cardiovascular hospitalizations and days in the hospital, lower rates of all-cause and cardiovascular mortality, and improved quality of life compared to patients with usual care only. Prescher S, Koehler K, Koehler F. e-Health in cardiology: remote patient management of heart failure patients. e-Journal of Cardiology Practice. 2020;18(26).
- The randomized, multicenter “Telemedical Interventional Management in Heart Failure II (TIM-HF2)” trial showed for the first time a significant improvement in all-cause mortality based on non-invasive remote management. Telemedicine patients had to spend fewer days in the hospital due to unplanned cardiovascular admissions - on average, 3.8 days compared to 5.6 days in the usual care group. In terms of all-cause mortality, out of 100 heart failure patients in the year of care, only eight patients of the remote management group (7.8 per 100 patient-years) died, while eleven patients died under the usual medical care conditions (11.3 per 100 patient-years). Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-57.
Quality Control
FDA-approved.
Technical Aspects/Integration
Bluetooth-enabled with vendor app. Compatible with wearable Fitbit fitness tracker. Weight capacity 400 lbs.
Procurement/Cost
$39.95-49.95; can be purchased from Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Indiehealth Digital Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights.
Indiehealth Digital Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
- Remotely managed patients recently hospitalized for heart failure had fewer unplanned cardiovascular hospitalizations and days in the hospital, lower rates of all-cause and cardiovascular mortality, and improved quality of life compared to patients with usual care only. Prescher S, Koehler K, Koehler F. e-Health in cardiology: remote patient management of heart failure patients. e-Journal of Cardiology Practice. 2020;18(26).
- The randomized, multicenter “Telemedical Interventional Management in Heart Failure II (TIM-HF2)” trial showed for the first time a significant improvement in all-cause mortality based on non-invasive remote management. Telemedicine patients had to spend fewer days in the hospital due to unplanned cardiovascular admissions - on average, 3.8 days compared to 5.6 days in the usual care group. In terms of all-cause mortality, out of 100 heart failure patients in the year of care, only eight patients of the remote management group (7.8 per 100 patient-years) died, while eleven patients died under the usual medical care conditions (11.3 per 100 patient-years). Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-57.
Quality Control
Has not been evaluated by the FDA.
Technical Aspects/Integration
Bluetooth-enabled with vendor app. Weight capacity 440 lbs.
Procurement/Cost
Under review.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Withings Body Digital Scale
Full descriptionHeart failure Heart Failure Connected meters EHR-adjacent clinician portal Smartphone
Digitally-connected scale to monitor patients’ weights.
Withings Body Digital Scale
Heart Failure Connected meters EHR-adjacent clinician portal Smartphone
Description
Digitally-connected scale to monitor patients’ weights.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
- Remotely managed patients recently hospitalized for heart failure had fewer unplanned cardiovascular hospitalizations and days in the hospital, lower rates of all-cause and cardiovascular mortality, and improved quality of life compared to patients with usual care only. Prescher S, Koehler K, Koehler F. e-Health in cardiology: remote patient management of heart failure patients. e-Journal of Cardiology Practice. 2020;18(26).
- The randomized, multicenter “Telemedical Interventional Management in Heart Failure II (TIM-HF2)” trial showed for the first time a significant improvement in all-cause mortality based on non-invasive remote management. Telemedicine patients had to spend fewer days in the hospital due to unplanned cardiovascular admissions - on average, 3.8 days compared to 5.6 days in the usual care group. In terms of all-cause mortality, out of 100 heart failure patients in the year of care, only eight patients of the remote management group (7.8 per 100 patient-years) died, while eleven patients died under the usual medical care conditions (11.3 per 100 patient-years). Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-57.
Quality Control
Has not been evaluated by the FDA.
Technical Aspects/Integration
Bluetooth-enabled with vendor app. Also WiFi-enabled. In addition to weight, the scale screen displays a trend of last 8 weigh-ins and automatically calculates BMI. Weight capacity 396 lbs.
Procurement/Cost
$49.95-59.95; can be purchased from Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021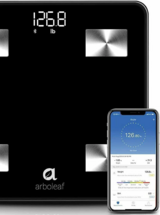
Arboleaf Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Arboleaf Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs to Arboleaf app via Bluetooth, which can then sync to Apple Health, Google Health and Fitbit apps. Weight capacity 400 lbs.
Procurement/Cost
$29.99; can be purchased on Amazon.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021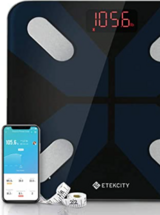
Etekcity Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Etekcity Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA-approved.
Technical Aspects/Integration
Syncs via Bluetooth 4.0 with free VeSync app, which then syncs data with Apple Health, Google Fit, Fitbit, and Samsung health. Weight capacity 400 lbs.
Procurement/Cost
$22.99-33.99; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Eufy Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Eufy Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs to EufyLife app via Bluetooth, which then syncs with Apple Health and Google Fit. Weight capacity 397 lbs.
Procurement/Cost
$44.99; can be purchased on vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Fitbit Aria 2 Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Fitbit Aria 2 Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA-approved.
Technical Aspects/Integration
Syncs via WiFi to Fitbit app on 200+ iOS, Android, and Windows devices as well as Fitbit watches and trackers. Weight capacity 400 lbs.
Procurement/Cost
$322.99; can be purchased on Amazon or vendor site and at Best Buy or Walmart.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021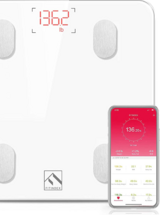
FITINDEX Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
FITINDEX Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs with FITINDEX app via Bluetooth, which can then sync data to Apple Health, Google Fit, Samsung Health, or Fitbit app. Weight capacity 396 lbs.
Procurement/Cost
$26.99-28.99; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
FitTrack Dara Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
FitTrack Dara Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA-approved.
Technical Aspects/Integration
Syncs to FitTrack Pro App via Bluetooth, which can then sync to other health apps including Apple Health and Google Health. Weight capacity 400 lbs.
Procurement/Cost
$89.95; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Garmin Index Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Garmin Index Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs to Garmin Connect or MyFitnessPal apps via WiFi with these compatible devices. Weight capacity 400 lbs.
Procurement/Cost
$149.99-189.99; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Greater Goods Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Greater Goods Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs to Greater Goods app via Bluetooth, which can then sync data to Fitbit, Google Fit, Apple Health, MyFitness Pal, and other apps. Weight capacity 400 lbs.
Procurement/Cost
$25.95-54.95; can be purchased on Amazon.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Omron Body Composition BCM-500 Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Omron Body Composition BCM-500 Connected Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA 510(k) pre-market notification.
Technical Aspects/Integration
Syncs via Bluetooth to OMRON HeartAdvisor on iOS or Android devices and the OMRON Health skill for Amazon Alexa-enabled devices. Weight capacity 330 lbs.
Procurement/Cost
$49.99-70.00; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
QardioBase 2 Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
QardioBase 2 Body Composition Connected Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs with Qardio app via Bluetooth 4.0 or WiFi, which can be used on iOS, Android, and Kindle devices. Syncs with Apple Health and Google Fit. Haptic feedback allows those with visual impairment to know when measurement has been taken. Has “pregnancy mode” for expecting mothers to track weekly progress and add pictures to their numbers. Weight capacity 396 lbs.
Procurement/Cost
$149.99; can be purchased on vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
RENPHO Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
RENPHO Body Composition Connected Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA-approved.
Technical Aspects/Integration
Syncs with Renpho app via Bluetooth 4.0. Premium model also connects to WiFi. Weight capacity 396 lbs.
Procurement/Cost
$29.99-45.99; can be purchased at Walmart, on Amazon, or on vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Rollibot Rollifit F8 Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Rollibot Rollifit F8 Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA-approved.
Technical Aspects/Integration
Syncs to Rolli-fit app on iOS and Android devices, which then syncs to Fitbit and Google Fit. Weight capacity 400 lbs.
Procurement/Cost
$24.99-69.99; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Tenswall Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Tenswall Composition Connected Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs to Tenswall app on iOS and Android devices. Weight capacity 400 lbs.
Procurement/Cost
$19.88-39.99; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Withings Body+ Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Withings Body+ Body Composition Connected Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
FDA 510(k) pre-market notification.
Technical Aspects/Integration
Syncs to Withings Health Mate app automatically via WiFi or Bluetooth. Allows users to track nutrition by setting a weight goal and daily calorie budgets. Has “pregnancy mode” for expecting mothers to track weekly progress. Weight capacity 396 lbs.
Procurement/Cost
$79.96-99.95; can be purchased on vendor site, Amazon, or at Apple, Bed Bath & Beyond, or Best Buy.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Withings Body Cardio Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Withings Body Cardio Body Composition Connected Scale
Heart Failure Connected meters Smartphone
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs to Withings Health Mate app automatically via WiFi or Bluetooth. Also measures heart rate. Has “pregnancy mode” for expecting mothers to track weekly progress, and “athlete mode” for athletes to measure their progress. Weight capacity 396 lbs.
Procurement/Cost
$119.96-149.95; can be purchased on vendor site, Amazon, or at Bed Bath & Beyond.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
Wyze Body Composition Connected Scale
Full descriptionHeart failure Heart Failure Connected meters Smartphone Smart watch
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Wyze Body Composition Connected Scale
Heart Failure Connected meters Smartphone Smart watch
Description
Digitally-connected scale to monitor patients’ weights and perform body composition analysis.
Clinical Indication
- Heart failure with reduced or preserved ejection fraction
- History of fluid overload
- Desired weight loss
- New exercise regimen
- Athletes
Evidence Base
Have not been independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Also measures heart rate. Syncs to Wyze app via Bluetooth, which can then sync to Apple Health and Fitbit apps. Weight capacity 400 lbs.
Procurement/Cost
$19.99-28.98; can be purchased on Amazon or vendor site.
Clinical Lead
Monique Tanna
Entry Author and Date
Monique Tanna, July 2020; Allison Hare, January 2021
HidrateSPARK Connected Water Bottles
Full descriptionHeart failure Heart Failure Connected objects Smartphone
Non-invasive fluid intake monitoring system.
HidrateSPARK Connected Water Bottles
Heart Failure Connected objects Smartphone
Description
Non-invasive fluid intake monitoring system.
Clinical Indication
- Heart failure
- History of fluid overload
- Kidney stones
Evidence Base
We sought to determine the accuracy of the Hidrate Spark™ smart water bottle in measuring 24-h fluid intake when used in a real life setting by an unaffiliated group of volunteers. We found that measurements taken through the bottle were within 3% of those measured by hand. This device has considerable potential as a behavioral aide to help patients with nephrolithiasis achieve high fluid intake and decrease stone risk. Future studies testing this device in a clinical setting are warranted and underway. Borofsky MS, Dauw CA, York N, Terry C, Lingeman JE. Accuracy of daily fluid intake measurements using a "smart" water bottle. Urolithiasis. 2018 Aug;46(4):343-348.
Quality Control
Not evaluated by the FDA.
Models
STEEL model is made of stainless steel; 3 model is made of plastic.Technical Aspects/Integration
“Hydration equation” calculator daily water goals using factors like age, height, weight, sex, elevation, and exercise. Syncs to HidrateSpark app, available on iOS and Android, via Bluetooth, which can then sync to fitness trackers. LED smart sensor glows and app sends text notifications to remind users when they should drink.
Procurement/Cost
STEEL is $64.99; 3 is $54.95. Both can be purchased on vendor site and Amazon, or at Apple, Target, or Walmart.
Entry Author and Date
Allison Hare, January 2021
ozmo Active Connected Water Bottle
Full descriptionHeart failure Heart Failure Connected objects Smartphone Smart watch
Non-invasive fluid intake monitoring system.
ozmo Active Connected Water Bottle
Heart Failure Connected objects Smartphone Smart watch
Description
Non-invasive fluid intake monitoring system.
Clinical Indication
- Heart failure
- History of fluid overload
- Kidney stones
Evidence Base
Not independently studied.
Quality Control
Not evaluated by the FDA.
Technical Aspects/Integration
Syncs via Bluetooth with Ozmo Water App, and fitness trackers from Fitbit, Garmin and Apple to help users track hydration.
Procurement/Cost
$69.99; can be purchased on Amazon.
Entry Author and Date
Allison Hare, January 2021Arrhythmia

AliveCor KardiaMobile Personal ECG Monitor + App
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors Smartphone
Personal ECG monitor that syncs with an app with AI-based arrhythmia detection.
AliveCor KardiaMobile Personal ECG Monitor + App
Arrhythmia Post-MI/Angina Biosensors Smartphone
Description
Personal ECG monitor that syncs with an app with AI-based arrhythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Bradycardia
-
Tachycardia
-
Premature ventricular contractions
-
Supraventricular ectopy
-
QTc monitoring
-
Self-monitoring in EP
Evidence Base
-
Site with 109 peer-reviewed articles in numerous categories in relation to Kardia device
-
Not tested/recommended for use with PPMs/ICDs
Quality Control
Device and app are FDA-cleared.
Models
-
KardiaMobile: contains one lead
-
KardiaMobile 6L: contains six leads
-
KardiaCare: Add-on software package that allows for detection of PVCs, supraventricular ectopy, and QTc length
Technical Aspects/Integration
-
Captures single lead ECG (30-second to 5-minute recordings) and provides instant analysis of various rhythms (AF, NSR, brady, tachy, unclassified/inconclusive). Sends data to mobile app via Bluetooth, where data is stored and can be emailed or sent via MPM to clinician.
-
Works with most Apple or Android smartphones and tablets
-
Not currently connected to Epic
Procurement/Cost
Available on vendor site or Amazon. KardiaMobile is $89, KardiaMobile 6L is $149, KardiaCare is $9.99 monthly.
Clinical Lead
Amaryah Yaeger, David Lin
Notes
Entry Author and Date
Amaryah Yaeger, April 2020; Allison Hare, February 2021
Arrhythmia-Monitoring BardyDx CAM patch
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Wearable device allowing for continuous ECG recording and arrythmia detection.
Arrhythmia-Monitoring BardyDx CAM patch
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
A prospective comparative study in which 50 patients wore the CAM and Holter monitor simultaneously over a limited 24-hour time period. The CAM significantly improved rhythm diagnosis and was rated as more comfortable and preferable to wear by patients. Smith WM., et al. Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours. American Heart Journal. March 2017.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
- Single patient use, continuous recording ambulatory ECG monitor that records for up to 14 days
- Uploads arrhythmia information to “BDxCONNECT”, patient/provider online portal
Procurement/Cost
Reimbursable under CPT codes for continuous cardiac monitoring up to 48 hours, 7 days, or 15 days.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021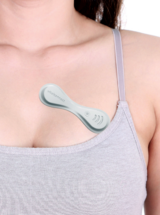
Arrhythmia-Monitoring VitalPatch
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Wearable device allowing for continuous ECG recording and arrythmia detection.
Arrhythmia-Monitoring VitalPatch
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Multivariate physiological telemetry from a wearable sensor can provide accurate early detection of impending rehospitalization with a predictive accuracy comparable to implanted devices. The clinical efficacy and generalizability of this low-cost noninvasive approach to rehospitalization mitigation should be further tested. Stehlik J, Schmalfuss C, Bozkurt B, Nativi-Nicolau J, Wohlfahrt P, Wegerich S, Rose K, Ray R, Schofield R, Deswal A, Sekaric J, Anand S, Richards D, Hanson H, Pipke M, Pham M. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization: The LINK-HF Multicenter Study. Circ Heart Fail. 2020 Mar;13(3):e006513.
Quality Control
Class 2 FDA device.
Technical Aspects/Integration
- Single-use and fully disposable
- 7-day wear duration with no charging required
- Contains a single-lead ECG, 3-axis MEMS accelerometer to detect motion, and thermistors to detect body temperature
Procurement/Cost
Under review.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
BioTel Arrhythmia-Monitoring Patches
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Wearable device allowing for continuous ECG recording and arrythmia detection.
BioTel Arrhythmia-Monitoring Patches
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Not clinically reviewed.
Quality Control
FDA-approved.
Models
- MCOT patch: Gathers data from the sensor via Bluetooth, then sends that ECG data via a wireless connection automatically. Contains rechargeable lithium battery.
- ePatch: Report is uploaded and available 24/7/365 through the dedicated HIPAA-compliant website. Up to 14 days of continuous monitoring. Contains rechargeable lithium battery.
Procurement/Cost
Under review.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
Biotricity Bioflux Mobile ECG Device
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Wearable device allowing for continuous ECG recording and arrythmia detection.
Biotricity Bioflux Mobile ECG Device
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Not clinically evaluated.
Quality Control
FDA 510(k) cleared.
Technical Aspects/Integration
- Single-unit mobile cardiac telemetry (MCT) device
- Records ECG data for up to 30 days
- Uses cellular IoT connectivity to sync data with Bioflux Clinic app that faces both patients and clinicians
Procurement/Cost
Under review.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
Cardiomo Arrhythmia-Monitoring Device
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Wearable device allowing for continuous ECG recording and arrythmia detection.
Cardiomo Arrhythmia-Monitoring Device
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Not clinically evaluated.
Quality Control
Awaiting FDA clearance.
Technical Aspects/Integration
- Uploads data to patient-facing app. AI engine detects for arrhythmias and sends alerts to the care team
- 7 days rechargeable battery
- Single patch can be reused again or by multiple patients
Procurement/Cost
Available for pre-order as of May 2021.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
ECGCheck
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
ECG monitoring system that can upload data to a clinician for analysis.
ECGCheck
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Description
ECG monitoring system that can upload data to a clinician for analysis.
Evidence Base
This technology enables a high quality single lead ECG to be recorded quickly and easily on a standard iPhone. The high sensitivity, specificity and accuracy of the algorithm, and widespread distribution of smartphones, make this device ideal for community screening. Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013 Apr 30;165(1):193-4.
Quality Control
FDA-cleared for over the counter or prescribed use.
Technical Aspects/Integration
Syncs with mobile app via Bluetooth, and is then synced to a secure cloud server where ECG signal is viewable by clinicians.
Procurement/Cost
$79.99; can be acquired via vendor site.
Entry Author and Date
Allison Hare, February 2021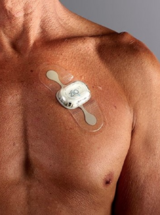
iRhythm Arrhythmia-Monitoring Zio Patch
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Wearable device allowing for continuous ECG recording and arrythmia detection.
iRhythm Arrhythmia-Monitoring Zio Patch
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
- Extended monitoring with the Zio Patch for ≤14 days is feasible, with high patient compliance, a high analyzable signal time, and an incremental diagnostic yield beyond 48 hours for all arrhythmia types. Turakhia MP, Hoang DD, Zimetbaum P, Miller JD, Froelicher VF, Kumar UN, Xu X, Yang F, Heidenreich PA. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013 Aug 15;112(4):520-4.
- Zio monitor is as accurate as an implanted pacemaker, the gold standard of monitoring, in detecting atrial fibrillation burden. Eysenck W, Freemantle N, Sulke N. A randomized trial evaluating the accuracy of AF detection by four external ambulatory ECG monitors compared to permanent pacemaker AF detection. J Interv Card Electrophysiol. 2020 Apr;57(3):361-369.
- The Zio patch has been shown to have a higher diagnostic yield for arrhythmia detection than Holter monitoring. An effectiveness study was carried out for the Zio patch, in which it was applied to 174 patients discharged from emergency departments, with an average age of 52.2 ± 21.0 years, with 55% of them being women. The most common indications among these patients included palpitations (44.8%), syncope (24.1%), and dizziness (6.3%), whereas the most common signs of arrhythmias were ventricular tachycardia (8.0%), atrial fibrillation (2.3%), bradyarrhythmias (2.9%), and unspecified arrhythmias (11.5%). These patients were asked to mail back the device at the end of 14 days or until they had symptoms to trigger an event. A total of 83 (about 48%) patients were recorded having more than 1 arrhythmia, with almost 10% being symptomatic at the time of their first arrhythmia. The median time for detection of first arrhythmia was found to be 1.0 days, with around one-half of the symptomatic patients not having any arrhythmia during their triggered events, exhibiting an overall diagnostic yield of 63.2%. PM Barrett, R Komatireddy, S Haaser, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(95):e11-7.
Quality Control
FDA 510(k) pre-market clearance.
Models
- Zio AT: For low-risk patients, which doesn’t provide alerts or daily reports
- Zio AT: For high-risk patients, which provides alerts and daily reports
Technical Aspects/Integration
- The patch can be worn for up to 14 days and provides relatively long-term monitoring of cardiac rhythms without the need of battery replacement or recharge over this time
- It also includes an event marker button that can be pressed when patients are symptomatic
- Single-use
- “ZioSuite” clinician portal shows reports and alerts for patients with patches in place
Procurement/Cost
~$360; available on vendor website.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
Samsung/Wellysis Arrhythmia-Monitoring S-Patch
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone Smart watch
Wearable device allowing for continuous ECG recording and arrythmia detection.
Samsung/Wellysis Arrhythmia-Monitoring S-Patch
Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone Smart watch
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Achieved 99.72% arrhythmia-detecting accuracy based on AI-powered models. Jeon E, Oh K, Kwon S, Son H, Yun Y, Jung ES, Kim MS. A Lightweight Deep Learning Model for Fast Electrocardiographic Beats Classification With a Wearable Cardiac Monitor: Development and Validation Study. JMIR Med Inform. 2020 Mar 12;8(3):e17037.
No clinical evidence as of January 2021.
Quality Control
Not FDA-approved.
Technical Aspects/Integration
- Compatibility
- Android 4.4 Kit Kat or higher
- iOS Version 10 or higher
- Transfer data via Bluetooth to smartphones. The diary menu allows for tracking symptom history during the test. Data can be automatically sent to the cloud portal immediately after the test has been completed.
- AI analysis-based cloud portal and dashboard allows faster arrhythmia detection for clinicians
Procurement/Cost
Not commercially available.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021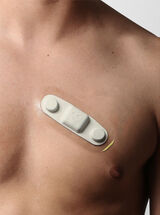
VivaLNK Arrhythmia-Monitoring Patch
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors Smartphone
Wearable device allowing for continuous ECG recording and arrythmia detection.
VivaLNK Arrhythmia-Monitoring Patch
Arrhythmia Post-MI/Angina Biosensors Smartphone
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Not clinically reviewed.
Quality Control
FDA-approved for ECG and heart rate recording.
Technical Aspects/Integration
- Up to 96 hours rechargeable
- BLE Network
- “IoT enabled”; uploads data to VivaLNK app through unclear mechanism
Procurement/Cost
$149; unclear procurement process.
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
Welch Allyn TACecg Wearable ECG Sensor
Full descriptionArrhythmia Arrhythmia Post-MI/Angina Biosensors
Wearable device allowing for continuous ECG recording and arrythmia detection.
Welch Allyn TACecg Wearable ECG Sensor
Arrhythmia Post-MI/Angina Biosensors
Description
Wearable device allowing for continuous ECG recording and arrythmia detection.
Clinical Indication
-
Atrial fibrillation
-
Unexplained syncope, near syncope, or episodic dizziness
-
Unexplained recurrent palpitations
-
To screen for asymptomatic ventricular premature beats or nonsustained ventricular tachycardia in a patient with hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, long QT syndrome, dilated or restrictive cardiomyopathy, congenital heart disease, or Brugada syndrome
-
To evaluate prognosis following acute coronary syndrome
-
To assess for silent myocardial ischemia in a patient with known or suspected coronary heart disease
Evidence Base
Proprietary algorithm effective in detecting atrial fibrillation and flutter with greater than 98% PPV and sensitivity of 96% based on tests conducted on databases available through PhysioNet (https://www.physionet.org/), following guidelines provided by ANSI/AAMI EC57: 2012.
Quality Control
Under review.
Technical Aspects/Integration
Records for up to 7 days.
Procurement/Cost
Entry Author and Date
Jonathan Wakim, December 2020; Allison Hare, January 2021
Cardiio Heart Rate Monitoring App
Full descriptionArrhythmia Arrhythmia Vital monitors Mobile applications Smartphone
App designed to assist patients with tracking, analyzing, and sharing heart rate measurements.
Cardiio Heart Rate Monitoring App
Arrhythmia Vital monitors Mobile applications Smartphone
Description
App designed to assist patients with tracking, analyzing, and sharing heart rate measurements.
Evidence Base
None for arrhythmia detection.
Quality Control
App listed for fitness purposes, not medical purposes - therefore exempt from FDA review.
Technical Aspects/Integration
- Users place the tip of their index finger on their mobile device’s camera, which then uses an algorithm to estimate heart rate and display PPG waveform. Also provides high-intensity circuit training exercises and personal dashboard that allows users to track their performance based on heart rate
-
Syncs to Apple Health
Procurement/Cost
Free on Apple App Store with option to subscribe for “pro” features.
Entry Author and Date
Allison Hare, March 2021
Elite HRV Heart Rate Monitoring App
Full descriptionArrhythmia Arrhythmia Vital monitors Mobile applications Smartphone
App designed to assist patients with tracking, analyzing, and sharing heart rate measurements.
Elite HRV Heart Rate Monitoring App
Arrhythmia Vital monitors Mobile applications Smartphone
Description
App designed to assist patients with tracking, analyzing, and sharing heart rate measurements.
Evidence Base
None for arrhythmia detection.
Quality Control
App listed for fitness purposes, not medical purposes - therefore exempt from FDA review.
Technical Aspects/Integration
- App syncs with heart rate sensors and provides user “biofeedback” based on heart rate variability
-
Integrates with Apple Health, Training Peaks, Heads Up Health, Final Surge, Strava, and Sport Tracks apps. User must also have a heart rate monitor that syncs with the app from list found here
Procurement/Cost
Vendor-produced “CorSense” heart rate sensor is available for $165 from vendor site. App itself is free on the Apple App Store and Google Play.
Entry Author and Date
Allison Hare, March 2021
Instant Heart Rate: HR Monitor App
Full descriptionArrhythmia Arrhythmia Vital monitors Mobile applications Smartphone
App designed to assist patients with tracking, analyzing, and sharing heart rate measurements.
Instant Heart Rate: HR Monitor App
Arrhythmia Vital monitors Mobile applications Smartphone
Description
App designed to assist patients with tracking, analyzing, and sharing heart rate measurements.
Evidence Base
None for arrhythmia detection.
Quality Control
App listed for fitness purposes, not medical purposes - therefore exempt from FDA review.
Technical Aspects/Integration
- Users place the tip of their index finger on their mobile device’s camera, which then uses an algorithm to estimate heart rate and display PPG waveform after 10 seconds. Also displays “heart rate activity zone calculator” which allows user to correlate their heart rate with physical activity level
- Syncs to Apple Health
Procurement/Cost
Free on Apple App Store, Google Play, and Windows Phone app store, but has optional paid subscription for workout coaching videos and “healthy living advice”.
Entry Author and Date
Allison Hare, March 2021
Boston Scientific MyLATITUDE ICD Interrogation App
Full descriptionArrhythmia Heart Failure Arrhythmia Mobile applications EHR-adjacent clinician portal Smartphone
App designed to assist patients with tracking, analyzing, and sharing ICD interrogation.
Boston Scientific MyLATITUDE ICD Interrogation App
Heart Failure Arrhythmia Mobile applications EHR-adjacent clinician portal Smartphone
Description
App designed to assist patients with tracking, analyzing, and sharing ICD interrogation.
Evidence Base
Under review.Quality Control
FDA-approved.
Technical Aspects/Integration
-
Patient-facing app which provides functionality checks for LATITUDE ICD communicator, answers to common ICD questions, and setup/troubleshooting support for LATITUDE ICD communicator
-
App syncs with communicator over WiFi or cellular networks
Procurement/Cost
Free for download on Apple App Store and Google Play.
Entry Author and Date
Allison Hare, April 2021
Medtronic ICD Interrogation Apps
Full descriptionArrhythmia Heart Failure Arrhythmia Mobile applications EHR-adjacent clinician portal Smartphone
Apps designed to assist patients with tracking, analyzing, and sharing ICD interrogation.
Medtronic ICD Interrogation Apps
Heart Failure Arrhythmia Mobile applications EHR-adjacent clinician portal Smartphone
Description
Apps designed to assist patients with tracking, analyzing, and sharing ICD interrogation.
Evidence Base
- The aim of the study was to assess the effectiveness of remote monitoring of implantable cardioverter-defibrillators and evaluation in an outpatient setting during 12-month follow-up. A total of 354 outpatient and 514 remote follow-up visits were conducted. Episodes of arrhythmias and device malfunctions were detected with similar frequency in outpatient visits and in remote visits. During the study period, patient sense of safety increased. More patients preferred joined remote and outpatient visits as the optimal healthcare model. As the patient survey showed, the greatest benefit of the CareLink network was fast intervention and an increased sense of safety. Maciąg A, Mitkowski P, Mazurek M, Kaźmierczak J, Nowak K, Grabowski M, Oręziak A, Kempa M, Bacior B, Gepner K, Chmielewska-Michalak L, Lenarczyk R, Kiedrowicz R, Fuglewicz A, Cacko A, Szwed H. Patient perspective and safety of remote monitoring of implantable cardioverter-defibrillators in the Polish Nationwide Multicenter Registry: the Medtronic CareLink network evaluation. Kardiol Pol. 2020 Nov 25;78(11):1115-1121.
- A large retrospective analysis was performed in the US of more than 95,000 patients who were enrolled in the CareLink database, using MyCareLink Smart. The analysis looked into the proportion of patients who adhered to the follow-up transmissions according to the clinic schedule. There were 48,016 patients assigned app-based remote follow-up, and 40,511 (84.4%) of them activated their devices for remote care. Adherence analysis was limited to 14,232 patients who activated their remote care and had at least 12 months of follow-up after activation. Of these patients, 89% were considered adherent, as per Heart Rhythm Society guidelines, as they had at least one more transmission within 3 months to 1 year after activation. There was no difference in adherence to follow-up in patients having a generator change or a de novo device. There was also no difference between men and women. The high percentages of adherence across all age groups suggest patients’ ability and desire to continue using remote care. Tarakji KG, Vives CA, Patel AS, Fagan DH, Sims JJ, Varma N. Success of pacemaker remote monitoring using app-based technology: Does patient age matter? Pacing Clin Electrophysiol. 2018 Oct; 41(10):1329-1335.
Quality Control
FDA-approved.
Models
- Medtronic MyCareLink Heart: Patients with Medtronic heart devices that have Bluetooth wireless telemetry can use this app to transmit telemetry data to their clinicians. Device and clinical alerts can be programmed on, so if there are any alerts the physician will receive notification of these outside of the usual follow-up regimen, with regular connectivity between the CIED and the patient’s device. These alerts include events related to lead impedance, low battery voltage, atrial tachycardia (AT)/AF burden, VT episodes, fast V rate during AT/AF, capture management and percentage V pacing
- Medtronic MyCareLink Smart: Pairs with Medtronic MyCareLink Smart Reader ICD interrogation device to send remotely-captured device interrogation data to clinician-facing portal
Technical Aspects/Integration
Data syncs to app via Bluetooth which then syncs over cellular/WiFi to clinician-facing portal. List of compatible patient mobile devices here.
Procurement/Cost
Apps are free on App Store and Google Play. Compatible interrogation reader can be prescribed to patients but has unclear cost information.
Entry Author and Date
Allison Hare, April 2021
Coag-Sense Connected Coagulation Lab Meter
Full descriptionArrhythmia Arrhythmia Connected meters EHR-adjacent clinician portal
Digital meter for coagulation lab monitoring.
Coag-Sense Connected Coagulation Lab Meter
Arrhythmia Connected meters EHR-adjacent clinician portal
Description
Digital meter for coagulation lab monitoring.
Clinical Indication
Patients on warfarin therapy requiring regular INR checks.
Evidence Base
The aim of this study was to determine if the Coagsense point-of-care (POC) instrument provides more reliable INR measurements than Coagucheck XS POC in comparison to the Stago laboratory instrument in different disease states. Patients were invited to study if they had an of INR 2.0 to 5.0 and had a medical history of antiphospholipid syndrome, hypercoagulable disorder, autoimmune condition, peripheral vascular disease, mechanical heart valve, atrial fibrillation, or deep vein thrombosis/pulmonary embolism/cerebrovascular accident history. Seventy-seven patients were enrolled. Coagsense correlated well (92% of INRs within 20% of Stago, 64% of INRs within 0.2 of Stago, overall INR bias of 0.1 or 4%). Six patients had greater than 20% POC INR bias, which could have resulted in 4 warfarin dosing errors. The average Coagucheck XS INR bias (0.46-1.3 INR) increased with each 0.5 increase in laboratory INR, whereas Coagsense bias remained stable (0.1-0.25) as INR increased up to 4.3. Two patients correlated well on Coagucheck XS but not Coagsense. Arline K, Rodriguez C, Sanchez K. Reliability of Point-of-Care International Normalized Ratio Measurements in Various Patient Populations. Point of Care. 2020; 19(1):12-18.
Quality Control
FDA 510(k) clearance.
Models
Technical Aspects/Integration
- Portable meter that mechanically detects clot formation to measure INR with Bluetooth and WiFi connectivity, enabling INR value transmission to clinical team
- WiFi and Bluetooth connectivity options send results to EHR systems, testing service providers, printers or POCT data management systems such as TELCOR or RALS
Procurement/Cost
Under review.
Entry Author and Date
Allison Hare, April 2021Hyperlipidemia
Curo Digital Lipid Panel Meters
Full descriptionHyperlipidemia Hyperlipidemia Connected meters
Curo Digital Lipid Panel Meters
Hyperlipidemia Connected meters
Description
Digital meters that enable patients to measure their lipid level panels remotely.Evidence Base
None.
Quality Control
FDA approved.
Models
-
Curo L5 Digital Cholesterol Meter
-
Description: Measures total cholesterol, triglycerides, HDL, LDL, LDL/HDL and non-HDL using 35 uL blood
-
Technical aspects/integration: Up to 500 panel results stored on device
-
Procurement/cost: Full kit (meter + 20 test strips) = $99, meter = $79, 10 test strips = $39.90 from vendor site
-
-
Curo L7 Digital Cholesterol Meter
-
Description: Measures total cholesterol, triglycerides, HDL, and LDL using 5 uL blood
-
Technical aspects/integration: Up to 200 panel results stored on device
-
Procurement/cost: Full kit (meter + 10 test strips) = $289, 10 test strips = $79 from vendor site
-
Technical Aspects/Integration
No Bluetooth or Wifi connectivity. Patient would need to manually message data to clinician for them to record or review it.
Entry Author and Date
Allison Hare, April 2021Diabetes
Accu-Chek Blood Glucose Monitoring Systems
Full descriptionDiabetes Diabetes Connected meters Smartphone Smart watch
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Accu-Chek Blood Glucose Monitoring Systems
Diabetes Connected meters Smartphone Smart watch
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
The performance of the Accu-Chek Mobile was evaluated both in the hands of a scientist and of diabetes patients. The designated comparative method was a hexokinase-based laboratory method (Architect ci8200). Diabetics (N = 88) with previous experience of self-testing were recruited for the study. Patient samples, containing glucose in concentrations mainly between ˜4 and ˜20 mmol/L, were analyzed in duplicates both on the Accu-Chek Mobile and with the comparative method. The patients answered a questionnaire about the ease of use of the meter. The meter yields reproducible readings, with an imprecision CV <5% as required by the American Diabetes Association (ADA). Of the glucose concentrations obtained by both the scientist and the patients, more than 95% of the individual results were within ± 20% of the comparative method, meeting the ISO 15197 accuracy goal, but not the stricter ± 10% ADA goal. Sachse D, Bolstad N, Jonsson M, Sæves I, Johansson CB, Delezuch W, Hagve M, Hardang IM, Isaksson HS, Ivarsson A, Lehto L, Keikkala E, Mattsson N, Ranta JK, Stavelin A, Sudmann AA, Varsi K. The Accu-Chek Mobile blood glucose monitoring system used under controlled conditions meets ISO 15197 standards in the hands of diabetes patients. Scand J Clin Lab Invest. 2012 Sep;72(5):374-9.
Quality Control
FDA 510(k) pre-market clearance.
Models
"Guide" model has a back-lit display, unlike "Guide Me" model.
Technical Aspects/Integration
Devices sync to mySugr app via Bluetooth, which is free with purchase of meters.
Procurement/Cost
"Guide Me" model is $14.99; "Guide" model is $29.99. Meters can be purchased from vendor site.
Entry Author and Date
Allison Hare, April 2021
AgaMatrix Jazz Wireless 2 Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters Smartphone
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
AgaMatrix Jazz Wireless 2 Blood Glucose Monitoring System
Diabetes Connected meters Smartphone
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
The results from the AgaMatrix product were as good or better than leading brands on the market, with 97% of results within ±15% or ±15 mg/dl of the lab reference in > 95% of trials. Klonoff DC, Parkes JL, Kovatchev BP, Kerr D, Bevier WC, Brazg RL, Christiansen M, Bailey TS, Nichols JH, Kohn MA. Investigation of the Accuracy of 18 Marketed Blood Glucose Monitors. Diabetes Care. 2018;41(8):1681-1688.
Quality Control
FDA-approved.
Technical Aspects/Integration
- Bluetooth-enabled blood glucose monitor which pairs with app that also allows user to track insulin usage, carb intake, and weight data over time
-
Compatible with AgaMatrix Diabetes Manager app, which visualizes blood glucose data over time and can send data to caregivers via push notifications
Procurement/Cost
$20.99 from vendor site.
Entry Author and Date
Allison Hare, April 2021
Biotel Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters EHR-adjacent clinician portal
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Biotel Blood Glucose Monitoring System
Diabetes Connected meters EHR-adjacent clinician portal
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
Under review.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
- Cellular-enabled blood glucose monitor which transmits data to patient- and provider-facing online portal
- Given cellular connectivity of device, no need for patient app download
Procurement/Cost
Annual subscription has monthly cost of $24.99.
Entry Author and Date
Allison Hare, April 2021
Contour Next One Smart Meter Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters Smartphone
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Contour Next One Smart Meter Blood Glucose Monitoring System
Diabetes Connected meters Smartphone
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
In the laboratory study, 100% (600/600) of combined results for all 3 test strip lots met ISO 15197:2013 Section 6.3 accuracy criteria. In the clinical study, among subjects with diabetes, 99.4% (327/329) of subject self-test results, 99.7% (331/332) of results obtained by study staff, 97.2% (309/318) of subject palm results, and 100% (330/330) of venous results met ISO 15197:2013 Section 8 accuracy criteria. Moreover, 97.6% (321/329) of subject self-test results were within ±10 mg/dl (±0.6 mmol/L) or ±10% of the YSI reference result. Questionnaire results indicated that most subjects considered the system easy to use. Christiansen, MP. A New, Wireless-enabled Blood Glucose Monitoring System That Links to a Smart Mobile Device: Accuracy and User Performance Evaluation. Journal of Diabetes Science and Technology. 2017;11(3):567-573.
Quality Control
FDA-approved.
Technical Aspects/Integration
- Digital glucose meter which syncs data to patient-facing app and has traffic light-style light system to easily convey to user whether their glucose level is within, nearing the limits of, or out of range
- Data syncs to CONTOUR DIABETES app. List of compatible devices here
Procurement/Cost
$6.99 for a monitor; $32.97 for 100 strips. Available for purchase from Amazon, CVS, Kroger, Rite Aid, Target, Walgreens, and Walmart.
Entry Author and Date
Allison Hare, April 2021
Dario Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters Smartphone
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Dario Blood Glucose Monitoring System
Diabetes Connected meters Smartphone
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
List of vendor’s internal research here.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
-
Device with built-in lance and disconnectable meter which can plug into smartphone and thus upload glucose data to Dario app
-
Dario app allows users to share glucose data with family, caregivers, or clinical team members. App also has hypoglycemia alert system with tagged GPS location which is sent to caregivers. Users can select either iPhone- or Android-compatible meter
Procurement/Cost
Covered under some insurance plans. “Basic package” of device + app is $30 monthly, “pro” package of device + app + personal coach is $40 monthly, “premium” package of device + app + personal coach + monthly check-in calls is $85 monthly. Discounted pricing if user signs up for 12 months.
Entry Author and Date
Allison Hare, April 2021
Dexcom G6 Continuous Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters EHR-adjacent clinician portal Smartphone Smart watch
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Dexcom G6 Continuous Glucose Monitoring System
Diabetes Connected meters EHR-adjacent clinician portal Smartphone Smart watch
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
76 participants with insulin-treated diabetes were enrolled at four U.S. sites as part of a larger study of G6 system performance. In-clinic visits for frequent comparative blood glucose measurements using a reference instrument (YSI) were conducted on days 1, 4–5, 7, and/or 10 of system use. Accuracy evaluation included the proportion of CGM values that were within ±20% of YSI reference value for glucose levels >100 mg/dL and ±20 mg/dL for YSI glucose levels ≤100 mg/dL (%20/20), the analogous %15/15 and %30/30, and the mean absolute relative difference (MARD) between temporally matched CGM and YSI values. Reprocessed data from 62 participants (25 adults and 37 children and adolescents of ages 6–17 years; 3532 YSI–CGM pairs) were analyzed. The G6 system's overall %20/20 was 93.9% (adults, 92.5%; children and adolescents, 96.2%), its %15/15 was 83.3% (adults, 78.3%; children and adolescents, 91.1%), and its MARD was 9.0% (adults, 9.8%; children and adolescents, 7.7%). Overall day-1 %20/20 accuracy was 92.2%, %15/15 was 81.5%, and MARD was 9.3%. Accuracy was maintained across 10 days of use and various glucose concentration ranges in both adults and children and adolescents. Shah V LL, Wadwa P, et al. Performance of a Factory-Calibrated Real-Time Continuous Glucose Monitoring System Utilizing an Automated Sensor Applicator. Diabetes Technology and Therapeutics. 2018;20(6).
Quality Control
FDA-approved.
Technical Aspects/Integration
-
A sensor continuously measures glucose levels just beneath the skin and sends data wirelessly to a display device through a transmitter. Customizable alerts when glucose levels fall out of range
-
Glucose data syncs with monitor to provide graph-based visualization of continuous glucose levels, and can send data to up to 10 people including clinical team members. Device compatibility checker here
Procurement/Cost
Medicare coverage details here.
Entry Author and Date
Allison Hare, April 2021
Eversense Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters Smartphone
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Eversense Blood Glucose Monitoring System
Diabetes Connected meters Smartphone
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
PRECISE II was a nonrandomized, blinded, prospective, single-arm, multicenter study that evaluated the accuracy and safety of the implantable Eversense CGM system among adult participants with T1D and T2D. The primary endpoint was the mean absolute relative difference (MARD) between paired Eversense and Yellow Springs Instrument (YSI) reference measurements through 90 days post-insertion for reference glucose values from 40 to 400 mg/dL. The primary safety endpoint was the incidence of device-related or sensor insertion/removal procedure-related serious adverse events (SAEs) through 90 days post-insertion. Ninety participants received the CGM system. The overall MARD value against reference glucose values was 8.8% (95% confidence interval: 8.1%–9.3%), which was significantly lower than the prespecified 20% performance goal for accuracy (P < 0.0001). Ninety-three percent of CGM values were within 20/20% of reference values over the total glucose range of 40–400 mg/dL. Clarke Error Grid analysis showed 99.3% of samples in the clinically acceptable error zones A (92.8%) and B (6.5%). Ninety-one percent of sensors were functional through day 90. One related SAE (1.1%) occurred during the study for removal of a sensor. Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, Denham DS, Atiee G, Bode BW, Walters SJ, Kelley L, Bailey TS. Diabetes Technology & Therapeutics. 2018;20(3):197-206.
Quality Control
FDA-approved.
Technical Aspects/Integration
- 90-day continuous glucose monitor with “on-vibe” body alerts to alert user when they become hypoglycemic and mobile app to monitor and transmit glucose data to other parties
-
Device compatibility data here
Procurement/Cost
Reimbursement information here.
Entry Author and Date
Allison Hare, April 2021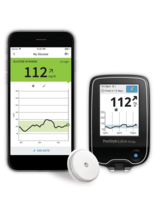
Freestyle Libre Connected Glucose Monitor
Full descriptionDiabetes Diabetes Connected meters EHR-adjacent clinician portal Smartphone Yes
Digital monitors that enable patients to monitor, analyze, and share blood glucose measurements.
Freestyle Libre Connected Glucose Monitor
Diabetes Connected meters EHR-adjacent clinician portal Smartphone Yes
Description
Digital monitors that enable patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
- An open-label, randomized, controlled study in adults with type 2 diabetes on intensive insulin therapy from 26 European diabetes centers aimed at assessing flash glucose sensing technology was conducted. Participants (N = 224) were randomized (1:2 respectively) to a control group (n = 75) that used self-monitoring of blood glucose (FreeStyle Lite™) or to an intervention group (n = 149) which used sensor glucose data (FreeStyle Libre™ Flash Glucose Monitoring System) for self-management over 6 months. At 12 months, time in hypoglycemia was reduced by 50% compared to baseline [−0.70 ± 1.85/24 h (mean ± standard deviation); p = 0.0002]. Nocturnal hypoglycemia was reduced by 52%; p = 0.0002. There was no change in time in range. Self-monitoring of blood glucose testing fell from a mean of 3.9 (median 3.9) times/day at baseline to 0.2 (0.0), with an average frequency of sensor scanning of 7.1 (5.7) times/day at 12 months, and mean sensor utilization was 83.6 ± 13.8% (median 88.3%) during the open-access phase. During this 6-month extension period no device-related serious adverse events were reported. Nine participants reported 16 instances of device-related adverse events (e.g. infection, allergy) and 28 participants (20.1%) experienced 134 occurrences of anticipated skin symptoms/sensor-insertion events expected with device use (e.g. erythema, itching and rash). Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of Flash Glucose-Sensing Technology for 12 months as a Replacement for Blood Glucose Monitoring in Insulin-treated Type 2 Diabetes. Diabetes Ther. 2017;8(3):573-586.
- Users performed a mean of 16.3 glucose checks per day, well above guidelines for self-monitoring. Estimated HbA1c reduced from 8.0% to 6.7% (64 to 50 mmol/mol) as scans increased from 4 to 48 per day. Time below 3.9, 3.1 and 2.5 mmol/L decreased by 15%, 40% and 49% from lowest to highest scan groups. Higher rates of scanning were linked to increased time in range and reduced time in hyperglycaemia. Dunn, T., et al. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycemic measures: A European analysis of over 60 million glucose tests. Diabetes Research and Clinical Practice. 2018;137:37-46.
Technical Aspects/Integration
-
Sensor which uses a thin, flexible filament inserted under the skin for up to 14 days to continuously monitor glucose levels. User checks glucose by scanning sensor with a handheld reader or smartphone
-
LibreLink app syncs with monitor to provide graph-based visualization of continuous glucose levels, and can send data to up to 20 people including clinical team members
Procurement/Cost
Most privately insured patients pay $10 - $75 per month.
Entry Author and Date
Allison Hare, April 2021
Livongo Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters EHR-adjacent clinician portal
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Livongo Blood Glucose Monitoring System
Diabetes Connected meters EHR-adjacent clinician portal
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
Under review.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
- Comprehensive program that provides connected diabetes monitor, unlimited strips, coaching, and advice
-
Diabetes meter sends glucose data to Livongo-based team through unclear mechanism
Procurement/Cost
Under review.
Entry Author and Date
Allison Hare, April 2021
One Drop Blood Glucose Monitoring System
Full descriptionDiabetes Diabetes Connected meters Smartphone Smart watch
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
One Drop Blood Glucose Monitoring System
Diabetes Connected meters Smartphone Smart watch
Description
Digital monitor that enables patients to monitor, analyze, and share blood glucose measurements.
Evidence Base
The One Drop Mobile app supports manual and passive tracking of self-care and glycated hemoglobin A1c (HbA1c). We assessed the HbA1c change of a sample of people with type 1 diabetes (T1D) or type 2 diabetes (T2D) using the One Drop Mobile app on iPhone and Apple Watch, and tested relationships between self-care tracking with the app and HbA1c change. In June 2017, we identified people with diabetes using the One Drop Mobile app on iPhone and Apple Watch who entered two HbA1c measurements in the app 60 to 365 days apart. We assessed the relationship between using the app and HbA1c change. There was a significant 1.36% or 14.9 mmol/mol HbA1c reduction (F=62.60, P<.001) from the first (8.72%, 71.8 mmol/mol) to second HbA1c (7.36%, 56.9 mmol/mol) measurement. Tracking carbohydrates was independently associated with greater HbA1c improvement (all P<.01). Osborn CY, van Ginkel JR, Marrero DG, Rodbard D, Huddleston B, Dachis J. One Drop | Mobile on iPhone and Apple Watch: An evaluation of A1c improvement associated with tracking self-care. JMIR mHealth and uHealth. 2017;5(11):e179.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
-
Bluetooth-enabled glucose meter which pairs with app that visualizes data and provides text-based coaching and predictive AI forecasting for glucose trends
-
Integrates with thousands of health-based apps, including Apple Health, FitBit, myfitnesspal, Withings, Amazon Alexa, Peloton, and more
Procurement/Cost
$30.99 monthly; can be purchased from vendor site.
Entry Author and Date
Allison Hare, April 2021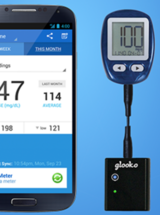
Glooko Connected Conduit for Unconnected Glucose Monitors
Full descriptionDiabetes Diabetes Connected meters Smartphone
Devices that add digital connectivity to unconnected glucose monitors.
Glooko Connected Conduit for Unconnected Glucose Monitors
Diabetes Connected meters Smartphone
Description
Devices that add digital connectivity to unconnected glucose monitors.
Evidence Base
Site states users have a 19.2% reduction in blood glucose levels, a 23.7% increase of in range glucose levels, and 15.1% reduction in hypoglycemic episodes after one year, but data is “on file” and not published.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
-
Device which syncs glucose data from unconnected glucose monitors to user smartphones; specifically to Glooko’s diabetes management app which allows users to also log food intake, view trends, and create medication reminders
-
List of compatible unconnected glucose meters here
Procurement/Cost
Entry Author and Date
Allison Hare, April 2021
Gluconext Connected Conduit for Unconnected Glucose Monitors
Full descriptionDiabetes Diabetes Connected meters Smartphone
Devices that add digital connectivity to unconnected glucose monitors.
Gluconext Connected Conduit for Unconnected Glucose Monitors
Diabetes Connected meters Smartphone
Description
Devices that add digital connectivity to unconnected glucose monitors.
Evidence Base
None.
Quality Control
Under review.
Technical Aspects/Integration
-
Retrieves blood glucose data from unconnected meters and transmits through Bluetooth to log book in smartphone. Two connection models: audio jack and infrared
-
List of compatible unconnected glucose meters here
Procurement/Cost
$29.99; can be purchased from vendor site.
Entry Author and Date
Allison Hare, April 2021
Medtronic InPen Connected Insulin Pen
Full descriptionDiabetes Diabetes Connected objects Smartphone
Digital insulin pen that enables patients to record and share their insulin injection information.
Medtronic InPen Connected Insulin Pen
Diabetes Connected objects Smartphone
Description
Digital insulin pen that enables patients to record and share their insulin injection information.
Evidence Base
This clinical trial is examining the impact that the InPen system has on patients’ clinical and psychosocial outcomes after 3 months of use, though no results have been posted as of April 2021.
Quality Control
FDA 510(k) pre-market clearance.
Technical Aspects/Integration
-
Smart pen that tracks users’ active insulin; calculates insulin doses; delivers long-acting insulin, missed dose, and blood glucose check reminders; delivers insulin expiration and temperature alerts; and compiles shareable reports to users’ healthcare teams
-
Connects to patient-facing InPen app via BlueTooth. Compatible with Novolog, Humalog, and Fiasp cartridges
Procurement/Cost
Minimum cost of $35 annually; users can submit form on vendor site to get information on insurance coverage of the device.
Entry Author and Date
Allison Hare, April 2021
Diabnext CLIPSULIN Connected Insulin Pen
Full descriptionDiabetes Diabetes Connected objects Smartphone
Digital insulin pen that enables patients to record and share their insulin injection information.
Diabnext CLIPSULIN Connected Insulin Pen
Diabetes Connected objects Smartphone
Description
Digital insulin pen that enables patients to record and share their insulin injection information.
Evidence Base
None.
Quality Control
Has not yet filed for FDA clearance.
Technical Aspects/Integration
-
Connects to most insulin pens, retrieves injection information and transmits it via Bluetooth to digital self-monitoring log-book on users’ smartphones
-
List of compatible insulin pens here
Procurement/Cost
$39.99; can be purchased from vendor site.
Entry Author and Date
Allison Hare, April 2021Wellness Tools

Apple Watches
Full descriptionWellness Tools Arrhythmia Wearables Smartphone Smart watch
Smart watch with features including heart rate and rhythm detection, pedometer, and sleep tracking.
Apple Watches
Arrhythmia Wearables Smartphone Smart watch
Description
Smart watch with features including heart rate and rhythm detection, pedometer, and sleep tracking.
Clinical Indication
Evidence Base
Apple Heart Study showed that among participants who received notification of an irregular pulse, 34% had atrial fibrillation on subsequent ECG patch readings and 84% of notifications were concordant with atrial fibrillation. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP; Apple Heart Study Investigators. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019 Nov 14;381(20):1909-1917.
Quality Control
ECG app is FDA approved to determine the presence of atrial fibrillation or normal sinus rhythm, but is not recommended for users with other known arrhythmias or users under the age of 22. Blood Oxygen app is not FDA-approved for clinical use.
Models
-
Series 6
-
Procurement/cost: $499 with GPS + cellular connectivity; $399 with GPS connectivity
-
Technical aspects/integration: Capable of detecting atrial fibrillation/normal sinus rhythm and providing a one-lead ECG reading through a one-lead ECG and third-generation optical heart sensor. Also hosts “Blood Oxygen app” which detects blood oxygenation through clusters of LED lights and photodiodes embedded in the watch backing. Syncs to Health app on iPhone and all Apple products via Bluetooth. Enabled with GPS and cellular connectivity
-
-
SE
-
Procurement/cost: $329 with GPS + cellular connectivity; $279 with GPS connectivity
-
Technical aspects/integration: Provides users notifications for high or low heart rates or irregular heart rhythms through a second-generation optical heart sensor. Syncs to Health app on iPhone and all Apple products via Bluetooth. Enabled with GPS and cellular connectivity
-
-
Series 3
-
Procurement/cost: $199 with GPS connectivity
-
Technical aspects/integration: Provides users notifications for high or low heart rates or irregular heart rhythms through an optical heart sensor. Syncs to Health app on iPhone and all Apple products via Bluetooth. Enabled with GPS connectivity
-
Technical Aspects/Integration
Procurement/Cost
Clinical Lead
Notes
Entry Author and Date
Jonathan Wakim, October 2020; Allison Hare, March 2021
Fitbit
Full descriptionWellness Tools Arrhythmia Wearables EHR-adjacent clinician portal Smartphone Yes
Smartwatches and wearables with functions including fitness, ECG, sleep, and SpO2 monitoring.
Fitbit
Arrhythmia Wearables EHR-adjacent clinician portal Smartphone Yes
Description
Smartwatches and wearables with functions including fitness, ECG, sleep, and SpO2 monitoring.
Evidence Base
Lubitz SA, Faranesh AZ, Atlas SJ, et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: The Fitbit heart study. Am Heart J. 2021;238:16-26. doi:10.1016/j.ahj.2021.04.003 Work underway to determine validity of atrial fibrillation detection.
Nelson BW, Allen NB. Accuracy of Consumer Wearable Heart Rate Measurement During an Ecologically Valid 24-Hour Period: Intraindividual Validation Study. JMIR Mhealth Uhealth. 2019;7(3):e10828. Published 2019 Mar 11. doi:10.2196/10828 Compared with the gold standard measurement method of an ambulatory ECG, the Fitbit Charge 2 provided acceptable heart rate accuracy (<±10%) across 24 hour periods and during a variety of activity intensities.
Models
-
-
Features: Detects electrodermal activity, detects episodes of atrial fibrillation, measures heart rate and variability, monitors SpO2, senses skin temperature, detects breathing rate, tracks sleep, measures activity through built-in GPS, and allows user to log mood, menstrual health, and more.
-
Procurement/cost: Can be purchased for $299.95 from vendor site.
-
-
-
Features: Measures heart rate and variability, monitors SpO2, senses skin temperature, detects breathing rate, tracks sleep, measures activity through built-in GPS, and allows user to log mood, menstrual health, and more.
-
Procurement/cost: Can be purchased for $229.95 from vendor site.
-
-
-
Features: Measures heart rate and variability, monitors SpO2, detects breathing rate, tracks sleep, measures activity, and allows user to log mood, menstrual health, and more.
-
Procurement/cost: Can be purchased for $179.95 from vendor site.
-
-
-
Features: Detects electrodermal activity, detects episodes of atrial fibrillation, measures heart rate and variability, monitors SpO2, senses skin temperature, detects breathing rate, tracks sleep, measures activity through built-in GPS, and allows user to log mood, menstrual health, and more.
-
Procurement/cost: Can be purchased for $179.95 from vendor site.
-
-
-
Features: Measures heart rate and variability, monitors SpO2, senses skin temperature, detects breathing rate, tracks sleep, measures activity, and allows user to log mood, menstrual health, and more.
-
Procurement/cost: Can be purchased for $149.95 from vendor site.
-
-
-
Features: Measures heart rate and variability, detects breathing rate, tracks sleep, measures activity, and allows user to log mood, menstrual health, and more.
-
Procurement/cost: Can be purchased for $99.95 from vendor site.
-
Technical Aspects/Integration
All devices sync to Fitbit smartphone app.
Entry Author and Date
Allison Hare, September 2021
CardiacSense Watch
Full descriptionWellness Tools Hypertension Arrhythmia Wearables
CardiacSense Watch
Hypertension Arrhythmia Wearables
Description
ECG and PPG signal-capturing watch that reportedly measures blood pressure and atrial fibrillation.Evidence Base
Link to studies relating to this product. One study of 24 volunteers demonstrated 99% reliability between ECG vs. CardiacSense watch detection of atrial fibrillation in the absence of motion-induced noise.
Quality Control
Not yet FDA approved.
Models
CardiacSense watch designed for continuous outpatient use; CardiacSense wristband designed for inpatient use to eliminate the need for telemetry and/or manual blood pressure or heart rate checks.
Technical Aspects/Integration
Leverages ECG and PPG signals to provide estimates of heart rate, heart rhythm, and blood pressure. Unclear how signal is transmitted to clinicians or integrated into clinical workflows.
Procurement/Cost
Not yet commercially available, but per site will be released in the front half of 2021.
Entry Author and Date
Allison Hare, March 2021
Withings Smartwatches
Full descriptionWellness Tools Arrhythmia Wearables Smartphone Smart watch
Hybrid smartwatches with functions including activity, heart rate, sleep apnea, and ECG tracking.
Withings Smartwatches
Arrhythmia Wearables Smartphone Smart watch
Description
Hybrid smartwatches with functions including activity, heart rate, sleep apnea, and ECG tracking.
Quality Control
ECG and pulse oximeter functions awaiting FDA approval as of March 2021.
Models
-
Steel HR
-
Tracks activity, heart rate, and sleep quality 24/7. Automatically detects when user is walking, running, swimming, or sleeping. “Smart Wake-Up” feature times alarm to user’s sleep cycle
-
Technical aspects/integration: Water resistant to 50 meters. Syncs to Withings’ “Health Mate” mobile application, to Apple Health, and to mobile phone’s GPS over BlueTooth. Can also sync to partnered fitness applications
-
Procurement/cost: $179.95, or $199.95 for “Sport Extra Metric” version with “Fitness Score” capability
-
-
ScanWatch
-
Tracks activity, heart rate, and sleep quality 24/7. Automatically detects when user is walking, running, swimming, or sleeping. “Smart Wake-Up” feature times alarm to user’s sleep cycle. Also contains ECG, multi-wavelength pulse oximeter, and “Respiratory Scan” feature which measures breathing frequency and moving during sleep to monitor for sleep apnea
-
Technical aspects/integration: Water resistant to 50 meters. Syncs to Withings’ “Health Mate” mobile application, to Apple Health, and to mobile phone’s GPS over BlueTooth. Can also sync to list of partnered fitness applications
-
Procurement/cost: Not yet released for commercial use. Priced at $279.95
-
-
Move
-
Automatically detects when user is walking, running, swimming, or sleeping. “Smart Wake-Up” feature times alarm to user’s sleep cycle
-
Technical aspects/integration: Water resistant to 50 meters. Syncs to Withings’ “Health Mate” mobile application, to Apple Health, and to mobile phone’s GPS over BlueTooth
-
Procurement/cost: $69.95
-
-
Move ECG
-
Tracks activity and contains ECG monitor. “Smart Wake-Up” feature times alarm to user’s sleep cycle
-
Evidence base: Live summary here
-
Technical aspects/integration: Water resistant to 50 meters. Syncs to Withings’ “Health Mate” mobile application and to Apple Health over BlueTooth
-
Procurement/cost: Not yet released for commercial use. Priced at $129.95
-
-
Pulse HR
-
Tracks activity, heart rate, and sleep quality 24/7. Automatically detects when user is walking, running, swimming, or sleeping. “Smart Wake-Up” feature times alarm to user’s sleep cycle
-
Technical aspects/integration: Water resistant to 50 meters. Syncs to Withings’ “Health Mate” mobile application, to Apple Health, and to mobile phone’s GPS over BlueTooth
-
Procurement/cost: $99.95
-
Notes
Withings “Data Hub” allows users without WiFi to send in their patient-generated data to a cellular-connected hub which can then transmit data to clinicians. All watches are compatible with the hub.
Entry Author and Date
Allison Hare, March 2021
Lose It! Diet & Nutrition App
Full descriptionWellness Tools Mobile applications Smartphone Smart watch
Lose It! Diet & Nutrition App
Mobile applications Smartphone Smart watch
Description
Calorie-counting and fitness-tracking app with embedded goal setting and weight loss insights.Evidence Base
Dietary self-monitoring is linked to improved weight loss success. Mobile technologies, such as smartphone applications (apps), might allow for improved dietary tracking adherence. The authors assessed the use of a popular smartphone app for dietary self-monitoring and weight loss by comparing it with traditional diet counseling and entry methods. Diet tracking and weight loss were compared across participants during an 8-week weight loss trial. Participants tracked intake using 1 of 3 methods: the mobile app "Lose It!", the memo feature on a smartphone, or a traditional paper-and-pencil method. App users (n = 19) recorded dietary data more consistently compared with the paper-and-pencil group (n = 15; P = .042) but not the memo group (n = 13). All groups lost weight over the course of the study (P = .001), and no difference in weight loss was noted between groups. Smartphone apps could represent a novel and feasible dietary self-monitoring method for individuals. Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self-monitoring, but not dietary quality, improves with use of smartphone app technology in an 8-week weight loss trial. J Nutr Educ Behav. 2014 Sep-Oct;46(5):440-4.
Models
Technical Aspects/Integration
Apps and devices Lose It! integrates with here.
Procurement/Cost
Free for download on the iOS App Store and the Google Play store with some in-app purchases for additional features available.
Clinical Lead
Notes
Entry Author and Date
Allison Hare, May 2021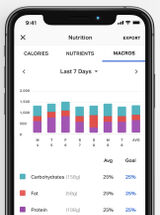
MyFitnessPal Diet & Nutrition App
Full descriptionWellness Tools Mobile applications Smartphone Smart watch
Food and activity log app with barcode scanning, goal-setting, and coaching capabilities.
MyFitnessPal Diet & Nutrition App
Mobile applications Smartphone Smart watch
Description
Food and activity log app with barcode scanning, goal-setting, and coaching capabilities.
Evidence Base
-
The aim of this study was to assess how individuals in naturalistic settings performed when recording their dietary intake in MyFitnessPal, and their usability experiences with the app. Adults not regularly using MyFitnessPal (N = 43) logged their dietary intake in the app for 4 consecutive days and completed two researcher-administered 24-h recalls collected based on the Automated Multiple Pass Method. Individuals omitted a mean of 18% (SD, 15) of food items, particularly energy-dense and nutrient-poor foods from MyFitnessPal records. Relative to 2-day 24-h recalls, 4-day MyFitnessPal records significantly underestimated mean energy intake by 1863 kJ (SD, 2952 kJ, P = 0.0002) and intake of all macronutrients. Although 80% of participants rated MyFitnessPal as easy to use, only 20% said they would continue use, citing challenges in matching foods, estimating portion size, and logging being time-consuming, as affecting motivation for long-term use. Large discrepancies in nutrient measurements from MyFitnessPal indicate suboptimal performance with using the app to record intake, particularly given food omissions in records and difficulties encountered with app usability relating to the food database and input of portion sizes. Stand-alone use of MyFitnessPal is therefore cautioned and guidance from dietitians is necessary to support use of nutrition apps in collecting accurate dietary data. Chen J, Berkman W, Bardouh M, Ng CYK, Allman-Farinelli M. The use of a food logging app in the naturalistic setting fails to provide accurate measurements of nutrients and poses usability challenges. Nutrition. 2019 Jan;57:208-216.
-
Thirty university students, including males and females, volunteered and recorded dietary intakes on paper-based food records and MyFitnessPal food records. MyFitnessPal tended to underestimate ingestion of nutrients probably due to inadequacies in their database. However, MyFitnessPal showed good relative validity, especially for energy and fibre. Its use, as well as other similar applications, should be encouraged, due to ease of assessing dietary information, although careful usage is recommended because of database gaps. Teixeira V, Voci SM, Mendes-Netto RS, da Silva DG. The relative validity of a food record using the smartphone application MyFitnessPal. Nutr Diet. 2018 Apr;75(2):219-225.
-
After carefully given instructions, 50 participants used MyFitnessPal to each complete a 4-day dietary record 2 times (T1 and T2), with 1 month in between T1 and T2. Nutrient intake values were calculated either manually, using the food composition database Nubel, or automatically, using the database coupled to MyFitnessPal. Cleaned MyFitnessPal values demonstrated strong correlations with Nubel for energy intake (r=0.96), carbohydrates (r=0.90), fat (r=0.90), protein (r=0.90), fiber (r=0.80), and sugar (r=0.79), but weak correlations for cholesterol (ρ=0.51) and sodium (ρ=0.53); all P values were ≤.001. A 5-10% power loss should be taken into account when correlating energy intake and macronutrients obtained with MyFitnessPal to an outcome variable, compared to Nubel. Overall, dietary analysis with MyFitnessPal is accurate and efficient for total energy intake, macronutrients, sugar, and fiber, but not for cholesterol and sodium. Evenepoel C, Clevers E, Deroover L, Van Loo W, Matthys C, Verbeke K. Accuracy of Nutrient Calculations Using the Consumer-Focused Online App MyFitnessPal: Validation Study. J Med Internet Res. 2020 Oct 21;22(10):e18237.
Technical Aspects/Integration
Integrates with HealthKit and 50+ additional apps to sync diet and fitness data.
Procurement/Cost
Free for download on the iOS App Store and the Google Play store.
Entry Author and Date
Allison Hare, May 2021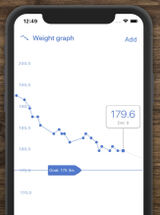
Noom Diet & Nutrition App
Full descriptionWellness Tools Mobile applications Smartphone Smart watch
Subscription-model health coaching with tracking and relevant content.
Noom Diet & Nutrition App
Mobile applications Smartphone Smart watch
Description
Subscription-model health coaching with tracking and relevant content.
Evidence Base
List to Noom-related publications here.
Technical Aspects/Integration
Noom syncs with the following apps.
Procurement/Cost
Monthly auto-recurring plan priced at $59/month, though longer plans are priced for a lower cost per month (i.e. the 4 month auto-recurring plan is priced at $129/month).
Entry Author and Date
Allison Hare, May 2021Communication & Care Delivery Tools
3M Littmann CORE Digital Stethoscope
Full descriptionCommunication & Care Delivery Tools Heart Failure Arrhythmia Post-MI/Angina Vital monitors EHR EHR-adjacent clinician portal Smartphone
3M Littmann CORE Digital Stethoscope
Heart Failure Arrhythmia Post-MI/Angina Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
Digital, noise-canceling stethoscope with arrhythmia pattern recognition.Technical Aspects/Integration
- Toggles between analog and amplified listening modes
- Volume can be magnified up to 40x
- Active noise cancellation
- AI arrhythmia detection
- Digital heart sound recording
Procurement/Cost
Can be purchased from vendor site from $349.
Entry Author and Date
Allison Hare, March 2021
Coala
Full descriptionCommunication & Care Delivery Tools Heart Failure Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Coala
Heart Failure Arrhythmia Post-MI/Angina Biosensors EHR-adjacent clinician portal Smartphone
Description
Hand-held, digital monitor for heart sounds, lung sounds, and ECG signals.Evidence Base
List of studies using Coala monitors.
Quality Control
FDA 510(k) cleared.
Technical Aspects/Integration
Connects to clinician-facing cloud portal and patient-facing app through Bluetooth.
Procurement/Cost
Can be prescribed as an Event Monitor (billed globally as CPT 93268) and/or used to follow patients long-term in RPM programs and billed monthly (CPT 99453, 99454, 99457 and 99458). Available for Home Enrollment, where it’s shipped directly to the patient from the vendor.
Entry Author and Date
Allison Hare, March 2021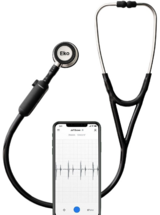
Eko Digital Stethoscopes
Full descriptionCommunication & Care Delivery Tools Heart Failure Arrhythmia Post-MI/Angina Vital monitors EHR EHR-adjacent clinician portal Smartphone
Eko Digital Stethoscopes
Heart Failure Arrhythmia Post-MI/Angina Vital monitors EHR EHR-adjacent clinician portal Smartphone
Description
Digital, noise-canceling stethoscopes with arrhythmia pattern recognition.Evidence Base
Living list of studies using Eko technology.
Quality Control
AI power recognition is FDA-cleared.
Models
-
Eko DUO ECG + Digital Stethoscope
-
Features:
-
Captures lung and heart sounds and ECG signals through a one-lead ECG
-
Patient holds device up to their chest without the need for clinician to listen through a stethoscope, and can thus be integrated into telemedicine workflows where a patient digitally transmits data to their clinician
-
Volume can be magnified up to 60x
-
-
Procurement/cost: Can be purchased from vendor site from $349
-
-
Eko CORE Digital Attachment
-
Extension that transforms analog stethoscopes into digital stethoscopes, with amplified listening, active noise cancellation, AI arrhythmia detection, and digital heart sound recording
-
Compatible with 3M™ Littmann®, ADC, WelchAllyn, MDF, and Medline stethoscopes
-
Procurement/cost: Can be purchased from vendor site from $199
-
Technical Aspects/Integration
Eko integrates with technology partners with an SDK/API, single sign-on (SSO), and EMR integration. Eko also has a clinician-facing dashboard and patient-facing app that can host and display ECG recordings and heart sounds.
Entry Author and Date
Allison Hare, March 2021Patient-Generated Health Data Platforms

Way to Health
Full descriptionPatient-Generated Health Data Platforms Hypertension Heart Failure Post-MI/Angina Obstructive Sleep Apnea EHR Smartphone Smart watch Yes
Way to Health
Hypertension Heart Failure Post-MI/Angina Obstructive Sleep Apnea EHR Smartphone Smart watch Yes
Description
Patient engagement platform for communication, remote monitoring device integration, and behavioral economics.Evidence Base
Portfolio of projects underway here.
Technical Aspects/Integration
Integrates with a number of vendors, including Epic, Omron, iHealth, Withings, FitBit, Misfit, Wisepill, Hydrate Spark, and AdhereTech. Additional device integrations can be added quickly on request.
Clinical Lead
Shivan Mehta, MD, MBA, MSHP

