Welcome to the Hambardzumyan lab
Dolores Hambardzumyan, PhD, MBA
Scientific Director, Brain Tumor Center
Professor, Department of Neurosurgery
Perelman School of Medicine
University of Pennsylvania
Background:
Gliomas are the most common primary brain tumors in both children and adults. Among these, glioblastoma is the most aggressive subtype and is considered one of the deadliest forms of human cancer. Despite intensive multimodal therapies, glioblastomas remain universally fatal. Recent advances in glioma biology have revealed that these tumors exist within a highly complex microenvironment composed of both neoplastic (tumor) and non-neoplastic (normal) cells. Research from our laboratory and others has shown that glioma growth is sustained by regulatory signals derived from this microenvironment. Consequently, brain tumors are viewed as complex cellular ecosystems where interactions between tumor and non-tumor components influence tumor initiation, progression, and resistance to therapy.
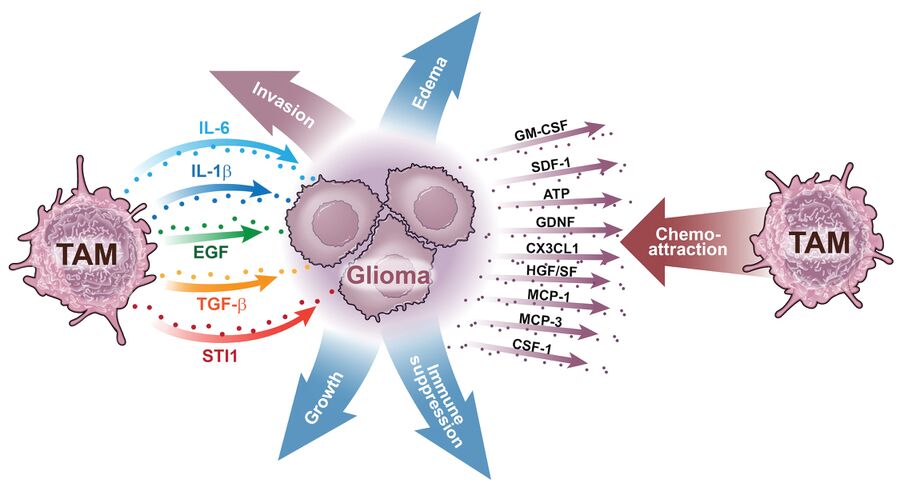
The most abundant non-neoplastic cell population within the glioblastoma microenvironment is the myeloid cell population. Various subsets of these cells are recruited into the tumor mass from the bloodstream, where they exert immunosuppressive properties and secrete growth factors and cytokines in response to signals from the tumor (Fig. 1). Although myeloid subsets are genetically stable, they exhibit diverse transcriptional programs in glioblastoma. Our research has demonstrated that the abundance, composition, and expression profiles of myeloid cells differ across various molecular subtypes of human glioblastoma, likely reflecting the genetic heterogeneity inherent to these tumors. These differences affect how myeloid subsets interact with both tumor cells and T cells, ultimately affecting the disease course and response to therapy.

Glioblastoma ecosystems are further stratified by their spatial architecture. Two major anatomical regions have been defined: the tumor core, which includes perivascular and hypoxic niches, and the tumor periphery, encompassing the infiltrative and invasive edge. The cellular heterogeneity across these regions underscores the clinical significance of spatially organized tumor-supportive niches and highlights the multidirectional interactions that sustain tumor growth (Fig. 2).
Mission:
In addition to our research on adult glioblastoma, our team is dedicated to advancing the understanding of pediatric high-grade gliomas (pHGG). We focus particularly on hemispheric pHGG and diffuse intrinsic pontine glioma (DIPG). Our research explores how the anatomical location of tumors and specific histone mutations shape the tumor microenvironment and influence responses to therapy.
Our laboratory examines how various myeloid subsets infiltrate tumors and interact with one another, tumor cells, and T cells. We utilize fresh patient samples, patient-derived organoid cultures, genetically engineered mouse models, and next-generation humanized mice for our research.
Research Areas:
The role of driver mutations in shaping the composition and function of tumor-associated myeloid subsets in adult glioblastoma
The impact of histone mutations and tumor location on the immune microenvironment of pediatric high-grade gliomas
Primary mechanisms of resistance to immune checkpoint inhibitors in glioblastoma
The role of tumor-associated macrophages and astrocytes in glioblastoma-associated cerebral edema
The role of mismatch repair gene mutations in mediating resistance to temozolomide and immune checkpoint inhibitors in adult and pediatric high-grade gliomas
Approaches:
We employ advanced profiling approaches, including single-cell RNA sequencing, spatial transcriptomics, and multiplex flow cytometry, in conjunction with genetically engineered mouse models (GEMMs) and patient tumor samples, to define immune cell states across human and mouse glioblastomas. Additionally, we employ 2-photon live imaging to track the infiltration dynamics of various myeloid subsets and their interactions with different cell types within tumors.
Vision:
Our ultimate goal is to identify and disrupt tumor-supportive niches as a strategy to design more effective therapies against pediatric and adult high-grade gliomas.


