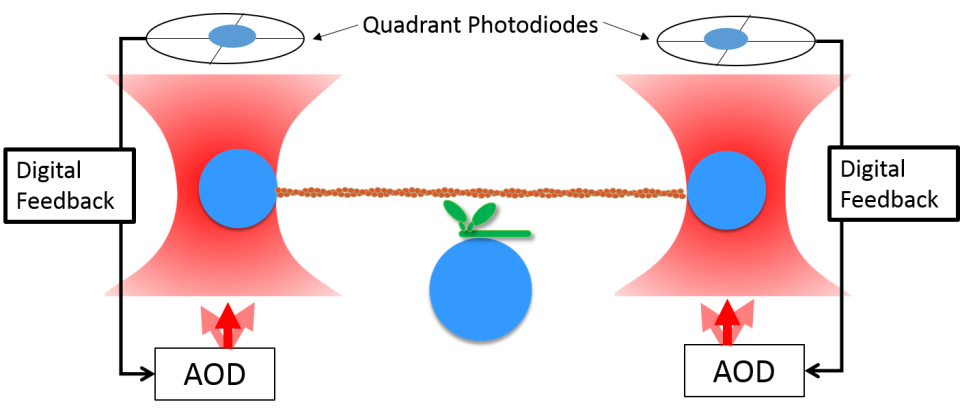
Optical trap with digital feedback.


Optical trap with digital feedback.
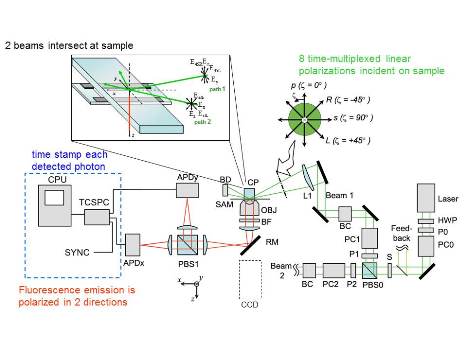
Our lab uses polarized Total Internal Reflection Fluorescence (polTIRF) microscopy to examine how the orientation of single molecules or subdomains change over time and with activity. This works by rigidly attaching a fluorescent probe with polarized emission to a protein of interest and measuring the emission intensity as a function of excitation and/or emission angle over time. Anti correlated changes in intensity at different angles, while total intensity remains constant, are used to calculate the orientation of the fluorphore, and therefore the protein, in three dimensions (X, Y, and Z). Observing these rotations over time provides valuable information about protein conformational changes and mechanisms.
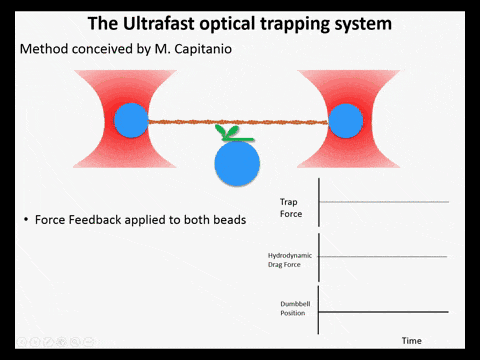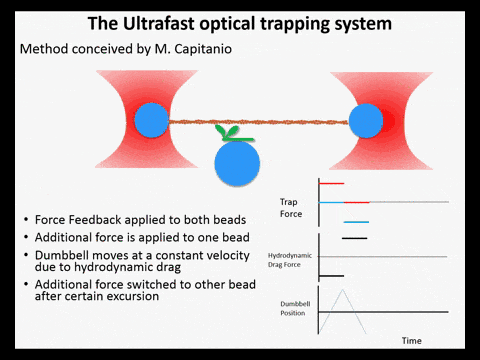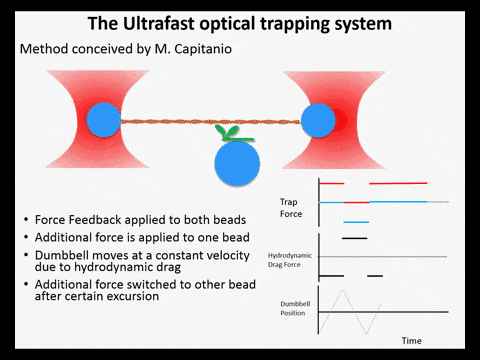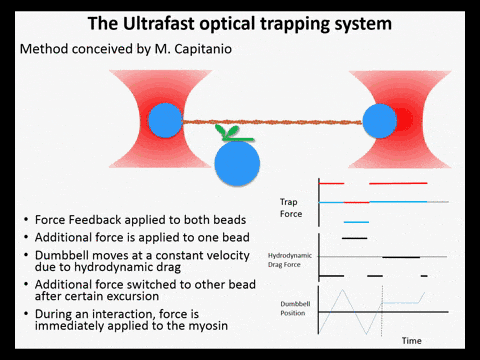
Optical trapping studies have provided much information about the mechanics and kinetics of motor proteins. Standard optical trapping methods require 5-15 ms to detect actin-myosin interactions. However, in the case of myosin, strong binding to the actin filament and force generation likely occurs within just a few milliseconds, making it difficult to study these transitions under load. We have developed an ultra-fast optical trapping technique that used a high-speed force feedback system to detect binding events within a few hundred microseconds. This allows the separate observation of actin binding and subsequent displacements of the actin filament by the myosin.
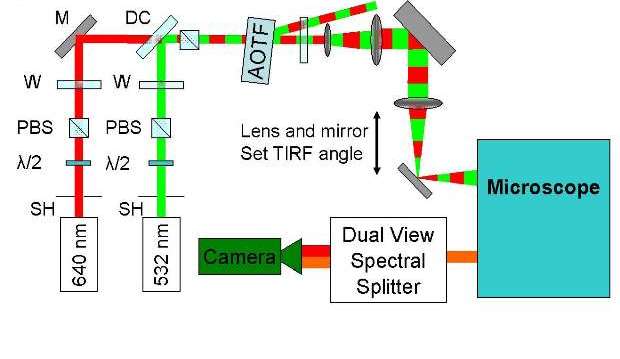
Alternating-laser excitation (ALEX) can be used with a 532 nm and 640 nm lasers to obtain Cy3 and Cy5 fluorescence intensities as well as the FRET (Forster Resonance Energy Transfer) signals between Cy3 and Cy5. Fluorescence intensities of Cy3 and Cy5 are separated by a dual view splitter and recorded with an electron multiplying charge-coupled device camera.
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser. | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team.