APPROACH # 2
We have developed a universal CAR T cell therapy that targets the pan-leukocyte marker CD45. To address the challenge of CD45-related on-target/off-tumor toxicity while preserving the essential functions of CD45, we identified the specific epitope on CD45 that is recognized by CAR T cells. By utilizing CRISPR adenine base editing, we introduced a function-preserving mutation to evade CAR T cell detection. This modification resulted in epitope-edited CD45 CAR T cells that are resistant to fratricide and effective against patient-derived cases of acute myeloid leukemia, B cell lymphoma, and acute T cell leukemia. Additionally, the epitope-edited hematopoietic stem cells (HSCs) were safeguarded from CAR T cell attacks, allowing them to engraft, persist, and differentiate in vivo, in contrast to traditional CD45 knockout cells. This innovative approach to ex vivo epitope editing in HSCs and T cells provides a safe and effective strategy for employing CD45-targeted CAR T cells and bispecific T cell engagers in the universal treatment of hematologic malignancies, with potential applications for other diseases that necessitate intensive hematopoietic ablation. See the graphics about engineering resistance for graft protection below and the full description below the image.
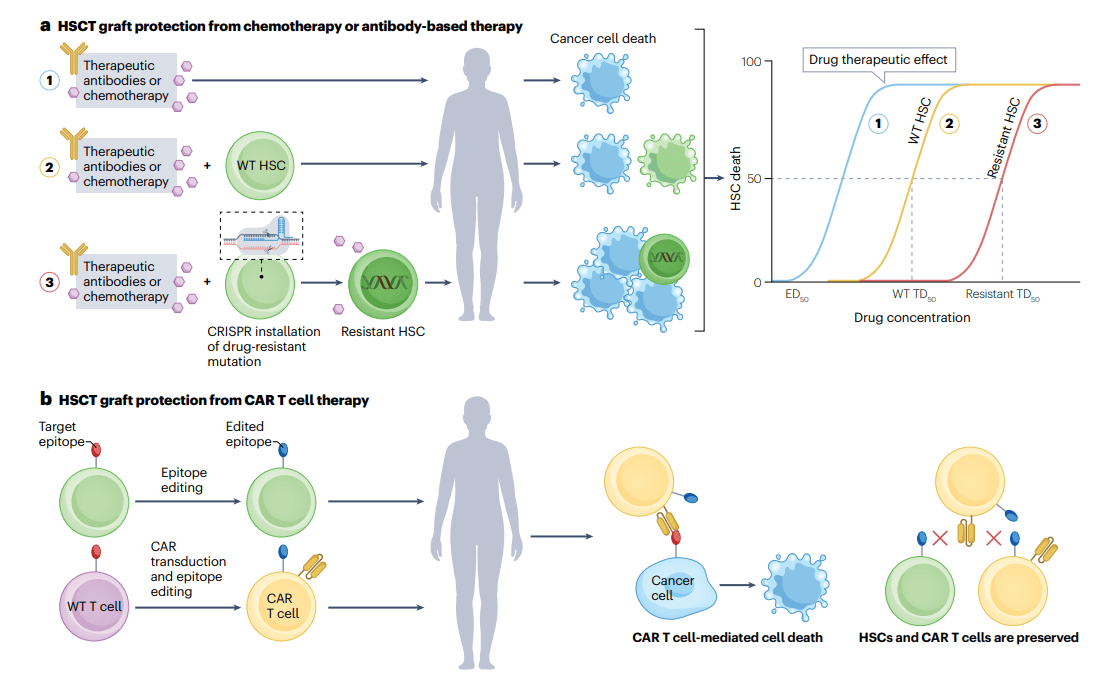
Engineering resistance for graft protection. a, Installing a drug resistant mutation in donor hematopoietic stem cells (HSCs) prior to HSC transplantation (HSCT) can increase the therapeutic index of chemotherapies or antibody-based therapies. After HSCT, administration of a drug can selectively eliminate any remaining cancer cells or wild-type (WT) host hematopoietic cells in the patient while sparing the donor graft with engineered resistance. The median toxic dose (TD50) of the drug for edited HSCs is higher than for WT HSCs, effectively shifting the therapeutic index of the drug. Editing donor HSCs makes the entire donor-derived hematopoietic system resistant to the drug;thus, post-HSCT administration of the drug is more tolerable for the patient. b, Epitope editing in both donor HSCs and chimeric antigen receptor (CAR) T cells prior to patient infusion serves two functions. First, cancer cells that have the WT target epitope remain susceptible to the edited CAR T cells, preserving CAR T cell effector function. Second, both the epitope-edited donor graft and epitope-edited CAR T cells are protected from CAR T cell-mediated killing, preserving the graft and preventing fratricide. This approach increases the repertoire of targetable cell-surface antigens in hematological cancers. ED50, median effective dose.



