The Second Britton Chance International Symposium on Metabolic Imaging and Spectroscopy
HONORING THE 105th BIRTHDAY OF BRITTON CHANCE
June 11-13, 2018
Rubenstein Auditorium, Smilow Center for Translational Research
Lecture Abstracts
Monday, June 11
Tuesday, June 12
Wednesday, June 13
Poster abstracts
LECTURE ABSTRACTS
JUNE 11, 2018
MONDAY – SESSION I: CANCER (1)
VIDEOS OF SESSION I
8:00 A.M.
Metabolic complexity in cancer cells and tumors
Ralph J. DeBerardinis, M.D., Ph.D.
Children's Medical Center Research Institute UT Southwestern Medical Center
Metabolism is dynamic and responds to a wide range of cell-intrinsic and cell-extrinsic factors. Many human diseases involve perturbations of metabolism at the cellular level, and in many cases normalizing the metabolic state has proven to be therapeutically valuable. Cancer is one such disease, in which factors both intrinsic and extrinsic to the malignant cell impact tumor biology and disease progression. In cancer, cell-intrinsic influences on metabolism include somatically-acquired mutations in oncogenes and tumor suppressor genes, many of which regulate metabolic activity. Cell-extrinsic factors include nutrient access, which may become limiting due to inadequate vasculature and intense fuel utilization, and metabolic interactions with stromal and immune cells. A major challenge in cancer metabolism research is to understand how these various factors culminate in the metabolic phenotype of an intact tumor, and ultimately to identify which altered pathways represent potential therapeutic targets. We have taken two parallel approaches to understand metabolic complexity in human cancer. The first uses a combination of multi-parametric imaging and intraoperative stable isotope infusions to assess metabolic fluxes in patients with solid tumors, and to compare fluxes between tumors and adjacent benign tissue. Genomic, histological and metabolic analysis of tumor samples allows us to correlate various intrinsic and extrinsic factors to specific aspects of the metabolic phenotype. The second approach uses standardized culture conditions to assess cell-intrinsic heterogeneity of metabolic preferences and dependencies in large panels of human cancer cell lines. This approach has uncovered liabilities associated with specific molecular subtypes of non-small cell and small cell lung cancer. I will discuss the application of these two approaches to metabolic heterogeneity in human cancer, with an emphasis on features relevant to intact tumors and therapeutic liabilities and the role of metabolic imaging in studying cancer metabolism.
Funding: National Cancer Institute (R35 CA220449), Howard Hughes Medical Institute (Faculty Scholars Program) and Cancer Prevention and Research Institute of Texas (RP160089)
8:40 A.M.
Optical metabolic probes characterize tumor life cycle stages
Nimmi Rammanujam, Ph.D.
Duke University
Residual tumor cells persist in dormancy for years before recurrence. Deregulated metabolism is a hallmark of cancer where tumors adapt their metabolism to survive hostile environments. The two primary axes are glycolysis and oxidative phosphorylation (OXPHOS) and alterations in either indicate poor prognosis. Understanding how tumors rewire their metabolism to enter dormancy and ultimately recur is critical in developing strategies to reduce recurrence and its associated morbidity. Towards this, we used optical imaging of two previously characterized fluorescent probes: 2-NBDG and TMRE to measure glucose uptake (glycolysis) and mitochondrial membrane potential (mitochondrial metabolism) respectively.
Tumor cells were extracted from an inducible genetically engineered mouse (GEM) model that exhibits key features of dormancy. In vivo, doxycycline (Dox) induces HER2 expression and tumor formation. Subsequent withdrawal of Dox causes oncogene down-regulation (similar to HER2-targeted therapies) and complete regression. FDG-PET has previously reported decreased uptake in regression. Cells from +Dox tumors in GEM mice were grown in vitro in 3D culture with Dox (active cells). Dox was removed from a subset of cultures for 2 and 4 days to model regression and 14 and 21 days to model dormancy. For recurrence, Dox was reintroduced to dormant cells to re-initiate HER2-overexpression.
Active and dormant cells demonstrated significantly greater glucose uptake than regressing cells (p<0.05 for both, n=10). Reactivated cells had significantly lower glucose uptake than all groups (p<0.05, n=10). Concurrently, regressing cells showed the highest MMP, followed by dormant cells, reactivated cells, and finally active cells (all p<0.05, n=10).
Our results demonstrate key metabolic shifts along a tumor’s life cycle. Decreased glucose uptake in recurrent vs. active cells suggests a shift in fuel source to other substrates to survive oncogene inhibition. This platform sets the stage to identify altered pathways key to recurrence and improve long-term patient outcome.
9:05 A.M.
New directions in nonlinear microscopy improve diagnosis of metatstaic potential
Warren, Warren, Ph.D.
Molecular imaging-the use of chemical signatures to image function instead of merely structure-promises to enable a new generation of clinical modalities that can revolutionize both diagnosis and treatment. In optics, our lab developed femtosecond pulse shaping two decades ago; today we know that the "killer application" is to access intrinsic nonlinear signatures that were not previously observable in tissue, such as excited state absorption, ground state depletion, and cross phase modulation. These methods permit high resolution imaging in scattering media, without any requirement that the imaging target generate fluorescence or other conventional light signatures. Our principal focus has been to improve biological imaging, for example to identify metastatic potential in thin melanomas (which cause the majority of melanoma deaths). The most promising application (and simplest to implement) is on pathology specimens. The fundamental challenge is that pathologists cannot discriminate between stage I melanomas which would never be dangerous, even if not cut out, and ones which will lead to metastatic cancer. Melanin, which has an exceedingly boring visible spectrum, turned out to have very rich dynamics in pump-probe microscopy, reflecting chemical composition, oxidation, aggregation, and chelation in ways that correlate extremely well with clinical outcome, based on retrospective studies. This approach is valid because of melanin's is its high stability-for example, eulemanin from 160 million year old fossils produces the same pump-probe decay as modern eumelanin. For example, we use this approach by correlating eumelanin imaging with sentinel node biopsy to evaluate tumor aggressiveness, which could revolutionize melanoma diagnosis by improving the pathology “gold standard.” We have shown this information is simply not contained in the conventional spectrum, even with hyperspectral analysis. Work with synthetic melanin models, squid eumelanin, cellular suspensions, and human biopsies lead to the same conclusions-we are seeing the consequences of the harsh intracellular environment and metabolic activity of the aggressive cancers. We show that the approach could also improve diagnosis both of ocular and vulvar melanoma, where simple incision (the “when in doubt, cut it out” approach) is less attractive in questionable cases.
MONDAY – SESSION II: METABOLISM AND FUNCTION (1)
VIDEOS OF SESSION II
10:00 A.M.
Coherent spacial frequency domain imaging (c-SFDI) of tissue metabolism
Bruce Tromberg, Ph.D.
Beckman Laser Institute, University of California, Irvine
10:25 A.M.
Advances in noninvasive assessment of organ oxygen consumption in humans by quantitative MRI
Felix Wehrli, Ph.D
University of Pennsylvania
Cellular respiration is central to energy production via phosphorylation of ADP to ATP. How much energy an organ utilizes can be assessed from a measurement of the oxygen consumed in this process (metabolic rate of oxygen, MRO2). Of particular interest is cerebral MRO2 (CMRO2), but much of the methodology for noninvasive assessment is applicable to other organ systems, such as skeletal and cardiac muscle. Recent advances in quantitative MRI make use of hemoglobin (Hb) magnetism, as Hb carries O2 to an organ and from there, by way of diffusion, to the cell’s mitochondria where it is metabolized. Quantitative MRI offers two entirely noninvasive approaches toward estimation of Hb saturation (HbO2) in O2 depleted blood, both of which build on hemoglobin paramagnetism. The first is a direct quantification of the blood’s bulk magnetic susceptibility (which scales linearly with deoxy-Hb concentration [dHb]). The second resorts to measurement of blood water proton T2, which is modulated by [dHb]. Together, with knowledge of blood flow rate (BFR), the two approaches yield MRO2 via Fick’s principle, in absolute physiologic units. This lecture briefly introduces the two methods, discusses recent work from the speaker’s laboratory in the brain and skeletal muscle in response to physiologic challenges, and delineates new applications for gauging oxygen metabolism of the placenta and fetus in utero in women during pregnancy.
10:50 A.M.
CEST enhanced metabolic imaging at ultrahigh fields
Ravinder Reddy
University of Pennsylvania
Chemical exchange saturation transfer (CEST)1-6 based MRI methods probe exchanging spin dynamics and provide endogenous as well as exogenous molecular contrast, which among other MR parameters depends on the concentration and pH of the solute spins. CEST methods require slow to intermediate exchange on the NMR time scale (chemical shift (&) > exchange rate (k)), and they primarily probe longitudinal magnetization exchange. Given the broad spectrum of exchange rates of amide, amine and hydroxyl protons on biologically important molecules, higher static magnetic field strengths allow a wider range of exchange rates to be probed. Consequently, the advent of whole body 7T MRI scanners has propelled research activity in CEST mediated molecular imaging and expanded the range of the metabolites able to be probed in vivo. The major advantage of the CEST method is that, depending upon the rates of exchangeable spins, it inherently has an order of magnitude or greater sensitivity advantage over conventional magnetic resonance spectroscopy (MRS) methods. In recent years, there has been an exponential growth in CEST MRI studies, and a variety of molecules including diamagnetic molecules, endogenous mobile proteins, liposomes, dendrimers, and complexes of paramagnetic ions have been shown to be potential CEST agents2-6. In this presentation, a brief outline of the basic principles, sensitivity advantages and recent developments of CEST methods, and their implementation in measuring endogenous metabolites in biological systems will be provided. Emerging applications of CEST MRI in studying neurological disorders7-10, cancer [11] and mitochondrial metabolism 12-13 will be outlined. Advantages and potential challenges of CEST MRI in quantifying the metabolites in vivo will be discussed.
RERERENCES:
1. S. Forsen and R. A. Hoffman (1963) J. Chem. Phys. 39:2892.
2. F. Kogan, H. Hariharan, R. Reddy (2013) Curr Radiol Rep 1:102.
3. A. D. Sherry and M. Woods (2008) Annu Rev Biomed Eng 10:391.
4. E. Vinogradov, A. D. Sherry, and R.E. Lenkinski (2013) J Magn Reson 229:15.
5. K. M. Ward, A. H. Aletras, R. S. Balaban (2000) J Magn Reson 143:79.
6. J. Zhou and P. C. van Zijl (2006) Prog. NMR Spectrosc 48:109.
7. K. Cai et al. (2012) Nat Med 18:302.
8. K. A. Davis et al. (2015) Sci Transl Med 7:309ra161.
9. P. Bagga et al. (2018) Sci Rep 8:2883.
10. R. Crescenzi et al. (2014) Neuroimage 101:185.
11. C. DeBrosse, et aL (2016) Sci Rep 6:19517.
12. M. Haris et al. (2014) Nat Med 20:209.
13. C. DeBrosse et al. (2016) JCI Insight 1, e88207.
MONDAY – SESSION III: CANCER (2)
VIDEOS OF SESSION III
2:10 P.M.
Molecular imaging and theranostics of cancer
Zaver M. Bhujwalla, M.Sc., Ph.D.
Johns Hopkins University School of Medicine
Advances in multi-modal noninvasive molecular and functional imaging are providing unique opportunities to expand our understanding of cancer – an understanding that is critical to developing effective and cancer specific treatments. The plasticity and adaptability of cancers, and the continually changing landscape they represent, highlight the importance of being able to noninvasively detect and understand the spatial and temporal heterogeneities of cancers. We have three broad interactive focus areas in my program that are being pursued using molecular and functional imaging: (1) investigating the tumor microenvironment (TME) to include understanding and imaging the extracellular matrix and mechanotransduction; (2) investigating the tumor – body interactions (the tumor macroenvironment, TMacE), to advance understanding of the cachexia syndrome; (3) development of theranostic agents to target cancer cells and cancer associated fibroblasts. The three focus areas are interactive with advances and insights from each area feeding into the other, and into the theranostics focus, with the intent of achieving precision medicine in cancer.
2:35 P.M.
Predicting cancer aggressiveness by optical redox imaging: feasibility and biological basis
He N. Xu, Ph.D., Lin Z. Li, Ph.D.
Perelman School of Medicine at the University of Pennsylvania
Although abnormal metabolism and intratumor heterogeneity have been established as two general hallmarks of cancer progression, the mitochondrial redox state and its intratumor heterogeneity have not been adequately studied nor linked to cancer transformation and progression to metastasis. Employing the optical redox/metabolic imaging (ORI/OMI) tools (including the 3D cryogenic Chance redox scanner and fluorescence microscope), we show in cell cultures, mouse models and clinical specimens the potential value of ORI indices for cancer diagnosis/prognosis and the importance of imaging intratumor metabolic heterogeneity with submillimeter resolution for predicting tumor progression to invasion and metastasis. We will also discuss some recent work in progress to understand why ORI indices may be prognostic from the perspectives of cancer microenvironment and genetics.
3:00 P.M.
Predicting breast cancer Residual Cancer Burden (RCB) during early phase neoadjuvant chemotherapy (NAC) by NIR spectral tomography (NIRST)
S. Jiang1, E. B. Bernhardt2, B. Batukbhai2, K. E. Muller2, J. Gui3, Xu Cao3, M. D. Chamberlin2, G. N. Schwartz2, K.D. Paulsen1, B. W. Pogue1, and P. A. Kaufman2
1Thayer school of Engineering, Dartmouth College, Hanover, NH; 2Dartmouth-Hitchcock Medical Center, Hanover, NH; 3Dartmouth Medical School, Hanover, NH
We have presented in our previous publications that NIRST imaging can differentiate outcomes of neoadjuvant therapy for pathologically complete response (pCR) patients from those with pathologically in-complete response (pIC), based upon the percentage change in tumor total hemoglobin (HbT) within the first cycle of treatment. In addition, pretreatment tumor HbT relative to the contralateral breast was also statistically significant in differentiating pCR from pIR. To further understand whether the residual cancer burden (RCB) can be predicted by NIRST in the earliest stages of NAC, the correlation between RCB index (from a reproducible pathologic analysis) and NIRST parameters has been investigated. In total, 16 women with locally advanced breast cancer were enrolled in this study. Patients had four NIRST imaging sessions prior, during (end of each of first and fourth treatment cycles) and post NAC. RCB scores were determined by a pathologist from surgical pathology slides. The RCB class was compared to NIRST HbT, oxygen saturation, and water, at each time point throughout NAC. To minimize baseline variations from inter-subject variability of breast density affecting the absolute hemoglobin and water ratios, relative to the pretreatment average of the contralateral breast were formed and used for statistical analysis. The statistical results show that the best predictor for final RBC class was the early change in total hemoglobin (HbT-Δ), which is the %change between the end of the first cycle to that before NAC started. The Pearson correlation coefficient of HbT-Δ was 0.71, with p-value=0.0019 for this coefficient using two-sided t-test. Given the good correlation with the RCB class, this study demonstrates the potential of NIRST as a validated imaging surrogate to predict NAC clinical outcome in the early treatment stage.
MONDAY – SESSION IV: MUSCLE, PANCREAS, AND OTHERS
VIDEOS OF SESSION IV
3:55 P.M.
Quantitative imaging of molecular mechanisms underlying islet hormone secretion
David W. Piston
Department of Cell Biology and Physiology, Washington University in St. Louis
The islet of Langerhans is the functional unit responsible for glucose-regulate secretion of insulin from β-cells and glucagon from α-cells, and thus plays a key role in blood glucose homeostasis. Chronic imbalance in the insulin/glucagon output of the islet is the defining trait of diabetes. Over the last 25 years, we have developed and used unique quantitative optical imaging methods and novel microfluidic devices to understand the multicellular mechanisms of islet function, and its role in the regulation of blood glucose under normal and pathological conditions. We have shown that the neurotransmitter dopamine plays an important role in islet function through autocrine and paracrine signaling between islet cells, which fine tunes hormone output via tonic inhibition of secretory activity. The dopamine receptors D3 and D2 are present and active in islet cells, and we are characterizing the complex interplay between the dopamine receptors that are active in the various islet cell types. To define a model for dopaminergic regulation of hormone secretion, we are using two-photon excitation metabolic imaging and confocal microscopy to assay responses triggered by selective activation of D2 or D3 receptors. We are also developing new methods for fast, high-content quantitative imaging of islet functions. We have combined light-sheet fluorescence microscope (dual view inverted Selective Plane Illumination Microscopy – diSPIM) and hyperspectral imaging (Image Mapping Spectrometer – IMS) to provide rapid acquisition of three-dimensional data sets of multiple biosensors in whole islets. This has allowed us to utilize mouse models combining multicolor Ca2+ and cAMP biosensors expressed in different islet cell types. This allows simultaneous monitoring of Ca2+ activity and cAMP levels in both α- and β-cells. Thus, we can measure putative paracrine interactions between the two cell types’ signaling pathways with sub-micron spatial and sub-second temporal resolution.
4:35 P.M.
Assessing skeletal muscle metabolism using near-infrared spectroscopy
Kevin McCully, Ph.D., FNAK, FACSM
Department of Kinesiology, University of Georgia, Athens
The ability to sustain submaximal exercise is largely dependent on the oxidative capacity of mitochondria within skeletal muscle, and impairments in oxidative metabolism have been implicated in many neurologic and cardiovascular pathologies. This presentation reviews studies which have demonstrated the utility of near-infrared spectroscopy (NIRS) as a method of evaluating of skeletal muscle mitochondrial capacity in human populations. Skeletal muscle mitochondrial capacity is assessed using the rate constant of the transition from exercising to resting metabolic states. Studies have shown the approach to be reproducible (coefficients of variation of 10-12%), independent of exercise intensity/mode, comparable to other methods (31P MRS and biopsy assessments), and sensitive to training induced changes. The method has been applied to a wide range of patient and athletic populations, and to a number of different muscle groups. The advantages to assessing mitochondrial capacity using NIRS generate great potential for the studying both clinical and sports populations.
5:00 P.M.
Human brown adipose tissue evaluated by near-infrared time-resolved spectroscopy
Takafumi Hamaoka1, Shinsuke Nirengi2, Sayuri Fuse1, Shiho Amagasa3, Toshiyuki Homma4, Ryotaro Kime1, Tasuki Endo1, Naoki Sakane2, Mami Matsushita5, Masayuki Saito6, Takeshi Yoneshiro7, Yuko Kurosawa1
1Tokyo Medical University, Department of Sports Medicine for Health Promotion, Japan: 2National Hospital Organization Kyoto Medical Center, Division of Preventive Medicine, Clinical Research Institute, Japan: 3Tokyo Medical University, Department of Preventive Medicine and Public Health, Japan: 4Daito Bunka University, Faculty of Sports and Health Science, Japan: 5Tenshi College, Department of Nutrition, Japan: 6Hokkaido University, Japan: 7UCSF Diabetes Center, University of California, Department of Cell and Tissue Biology, USA
Human brown adipose tissue (BAT) has been evaluated using 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) with computed tomography (CT). However, FDG-PET/CT has several limitations such as radiation exposure, which make repeated measurements difficult. Near-infrared time-resolved spectroscopy (NIRTRS) is a method of noninvasively quantifying the total hemoglobin concentration [total-Hb]. As BAT has abundant capillaries, NIRTRS should be able to assess BAT density by measuring indicators for hemoglobin concentration. The [total-Hb], an index of tissue vasculature, was determined using NIRTRS in the supraclavicular region adjacent to BAT under thermoneutral conditions. The BAT activity was quantified by calculating the maximal standardized FDG uptake value (SUVmax) in the supraclavicular region using FDG-PET/CT with a standardized protocol. NIRTRS parameters and SUVmax were compared in 29 healthy men. The [total-Hb] was significantly correlated to BAT activity (r = 0.75, P < 0.01) in the supraclavicular region close to BAT deposits. The result of our study suggests that the [total-Hb] in the supraclavicular region is a specific index of BAT density in humans. We will also discuss the usefulness and application of NIRTRS measurements for human BAT density evaluations.
References: Nirengi et al., Obesity 23(5):973-980, 2015.
5:15 P.M.
The RunMan legacy: measuring muscle metabolism 30 years later
Maria Angela Franceschini1, Parisa Farzam1, Pamela Anderson2, Daniel Wiese2, Alessandro Babini2
1Optics at Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02129, USA 2Dynometrics, Inc., Cambridge, MA 02139, USA
Thirty years ago Britton Chance first envisioned a “user-wearable hemoglobinometer for measuring the metabolic condition of a subject” and started the field of NIRS to measure skeletal muscles physiology. After three decades, skeletal muscles can be measured everywhere by anyone with wearable wireless devices, like the Humon H. By using more sophisticated devices, like the MetaOx, in addition to hemoglobin, we can quantify blood flow and oxygen metabolism during exercise. We used these two devices to monitor the exercising muscles of 17 athletic individuals while subjects performed an incremental test on a stationary cycle ergometer. Blood lactate concentration was measured at the end of each increment with a lactate analyzer. The optically measured hemodynamic and oxygen metabolism parameters were able to accurately predict the athletes’ lactate threshold power. These new devices hold promise for optimizing athletes’ training regimens. The authors have commercial interests in the technology used.
VIDEO OF THE BIOSTATISTICS WORKSHOP
5:35
Statistics, rigor, and reproducibility
Mary E. Putt
University of Pennsylvania
Multiple comparisons and false discovery rate using metabolic imaging data
Huaqing Zhao, Ph.D.
Temple University
NIH defines scientific rigor and reproducibility as the strict application of the scientific method to ensure robust and unbiased experimental design, methodology, analysis, interpretation and reporting of results. Improper use of statistical methods contributes to ‘irreproducible’ results. This talk reviews recent literature to illuminate common problems with statistical reproducibility and particularly problems arising from the use of p-values to determine 'statistical significance'. Specific problems resulting from low statistical power and multiple testing will be described. Study designs with low statistical power obviously have poor ability to detect effects of interest. It is less well known that ‘statistically significant findings’, from studies with low statistical power, represent extreme values. As a result, follow-up studies will often fail to replicate these large effects, particularly when replication is defined as p<.05. A further problem with reproducibility, particularly in imaging studies, arises from the problem of multiple hypothesis testing. We review these problems and some advances in statistical methodologies in imaging involving the problem of multiple comparisons.
JUNE 12, 2018
TUESDAY – SESSION I: METABOLISM AND FUNCTION (2)
VIDEOS OF SESSION I
8:00 A.M.
Towards understanding hydrogen peroxide in redox biology
Helmut Sies, M.D., Ph.D.
Institute for Biochemistry and Molecular Biology I, and Leibniz Research Institute for Environmental Medicine, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
Historical: At its heydays, Britton (called ‘BC’) Chance's Johnson Research Foundation (JF) was the center of mitochondriology, in the forefront of biophysical methodology using fluorescence and absorbance measurements noninvasively. Also, the microsomal CO-binding pigment, later known as cytochrome P450, was discovered at the JF. At Theodor Bücher’s lab in Munich, working with the Rapidspektroskop in organ spectrophotometry, I used the near-infrared charge transfer band of catalase Compound I to identify hydrogen peroxide as a normal aerobic metabolite, which was published together with BC (1). Ensuing joint work with Nozomu Oshino at the JF led to further insight, and mammalian hydroperoxide metabolism came into larger focus, together with Alberto Boveris (2).
Current. Hydrogen peroxide emerged as a central redox signaling molecule (3). Generation, transport and capture of H2O2 in biological settings as well as their biological consequences can now be addressed, using novel methodology. Basal physiological intracellular concentration of hydrogen peroxide is at around 10 nanomolar, a condition denoted as oxidative eustress. High (supraphysiological) hydrogen peroxide leads to disrupted redox signaling and damage to biomolecules, oxidative distress. Powerful oxidative stress response systems (e.g. Nrf2, NF-kB) counteract oxidative challenge. Spatiotemporal patterns of hydrogen peroxide, such as in nanodomains, and crosstalk with calcium-dependent pathways are integral to the ‘Redox Code’ (4).
(1) Sies H, Chance B (1970) FEBS Lett 11:172-176
(2) Chance B, Sies H, Boveris A (1979) Physiol Revs 59:527-605
(3) Sies H (2017) Redox Biol 11:613-619
(4) Jones DP, Sies H (2015) Antioxid Redox Signal 23:734-746
8:40 A.M.
Spatial and physical properties of mitochondrial oxidative phosphorylation
Douglas Wallace, Ph.D.
The Children's Hospital of Philadelphia
9:05 A.M.
Multiparametric, label-free, high resolution optical imaging for monitoring mitochondrial metabolism and dynamics
Irene Georgakoudi, Ph.D., Zhiyi Liu, Dimitra Pouli, Carlo Alonzo, and Kyle Quinn
Tufts University
The ability to monitor subcellular functional and structural changes associated with metabolism is essential for understanding tissue development and disease progression. However, established methods are either destructive or in need of exogenous contrasts. Here, we present in a systematic manner a quantitative approach, which can detect both functional and structural metabolic information non-invasively with subcellular resolution, relying on endogenous two-photon excited fluorescence from two coenzymes, NADH and FAD. We perform multi-parametric analysis comprising of redox ratio, NADH fluorescence lifetime and mitochondrial clustering, within intact cells and 3D tissues undergoing various metabolic perturbations, including glycolysis and glutaminolysis, extrinsic and intrinsic mitochondrial uncoupling, and fatty acid oxidation and synthesis. We demonstrate that the extraction of optical biomarkers provides complementary insights into the underlying biological mechanisms, and can serve as a resource that enables sensitive, label-free identification of metabolic perturbation pathways and characterization of the heterogeneity of the elicited responses with single cell resolution.
9:30 A.M.
Imaging metabolic plasticity in breast cancer models via redox enzyme systems
V. Krishnan Ramanujan, Ph.D.
Cedars Sinai Medical Center, Los Angeles, CA, USA
Tremendous advancements in anatomical imaging (mammography, ultrasound etc.,) in breast cancer (BC) screening and early detection have reduced BC-associated mortality significantly. However, increased detection sensitivity also leads to critical issues in the clinical management of breast cancer. For instance, an estimated 30% of all breast cancer cases are considered to be over-diagnosed and over treated. A critical problem in the management of breast cancers is to discriminate those patients that would benefit from cytotoxic drugs or anti-targeted therapy from those that would not. It is now being increasingly realized that additional information from molecular imaging and other physiological/metabolic aspects of tumors can benefit patient stratification. Towards this direction, it will be of immense value and significance to identify novel, tumor-specific metabolic read outs that can augment the current repertoire of early detection toolbox. This talk will focus on some of our laboratory studies addressing these clinical bottlenecks. We will present imaging-based metabolic phenotyping of breast cancer lesions using three-dimensional mammary tumor organoids that we have developed in our laboratory. We will also discuss the role of redox enzyme systems in modulating metabolic plasticity in these 3D breast cancer models and present preliminary imaging results that can inform us in breast cancer diagnosis and decision-making for more effective treatments.
9:55 A.M.
Monitoring diabetic wound metabolism through in vivo multiphoton microscopy
Jake D. Jones, Hallie E. Ramser, Alan E. Woessner, Kyle P. Quinn
Department of Biomedical Engineering, University of Arkansas, Fayetteville, Arkansas 72701
Non-healing wounds, such as diabetic foot ulcers, are challenging to diagnose and treat due to their numerous etiologies and the variable efficacy of wound care products. There is a critical need to develop new diagnostic technologies and quantitative biomarkers that are sensitive to specific wound characteristics. Multiphoton microscopy (MPM) techniques are well-suited for 3D skin imaging and are capable of non-invasively detecting autofluorescence from metabolic cofactors (NADH and FAD) without the need for exogenous dyes. The objective of this study was to demonstrate the ability to monitor the metabolism of individual full-thickness skin wounds in vivo using a combination of rapid MPM acquisition and image processing. Full-thickness excisional wounds were produced in diabetic (streptozotocin-induced) and control C57BL/6J mice (n=7 mice/group). Using MPM, we isolated and measured an optical redox ratio of FAD/(NADH+FAD) autofluorescence at the wound edge to provide 3D maps of cellular metabolism over a 10-day period from each mouse. A significant decrease in the optical redox ratio of the epidermis in both groups was observed between days 1 and 3 (p<0.003) and days 1 and 5 (p<0.005). By day 10, the epithelial redox ratio at the wound edge in the nondiabetic group had significantly increased (p<0.03) relative to days 3 and 5, while the diabetic mice displayed no significant temporal change. Ki-67 staining and wound closure rates indicate optical redox ratio and NADH lifetime measurements are correlated with the relative rates of keratinocyte proliferation and migration during healing. These findings demonstrate that keratinocytes at the edge of diabetic wounds remain in a proliferative state at later time points compared to control wounds. Our work demonstrates label-free MPM offers potential to provide non-invasive optical biomarkers associated with different stages of skin wound healing, which may be used to detect impaired healing and guide treatment.
TUESDAY – SESSION II: BRAIN
VIDEOS OF SESSION II
10:40 A.M.
fNIRS in 2023
David Boas, Ph.D.
Boston University
In 1993, four papers were published demonstrating that human brain activity could be measured with Near Infrared Spectroscopy (NIRS). One of those papers was from Britton Chance. This gave birth to the field of functional NIRS (fNIRS), which today at 25 years of age is still growing strongly with over 400 papers published in the field in 2014, supported by a Society for fNIRS and Facebook group with over 400 and 800 members respectively.
B. Chance, Z. Zhuang, C. UnAh, C. Alter, and L. Lipton, “Cognition-activated low-frequency modulation of light absorption in human brain.,” Proceedings of the National Academy of Sciences of the United States of America 90(8), 3770–3774, Proceedings of the National Academy of Sciences of the United States of America (1993) [doi:10.1073/pnas.90.8.3770].
11:10 A.M.
Non-invasive optical hemodynamic monitoring in critically brain-injured patients
Wesley B. Baker1,2, Ramani Balu3, Lian He4, Venkaiah C. Kavuri4 Lin Wang4, David R. Busch4, Olivia Amendolia5, Francis Quattrone5, Suzanne Frangos5, Eileen Maloney-Wilensky5 Elizabeth Mahanna1 Arjun G. Yodh4, W. Andrew Kofke1
1Department of Anesthesiology and Critical Care, University of Pennsylvania; 2Division of Neurology, Department of Pediatrics, Children’s Hospital of Philadelphia; 3Department of Neurology, University of Pennsylvania; 4Department of Physics and Astronomy, University of Pennsylvania; 5Department of Neurosurgery, University of Pennsylvania
INTRODUCTION: Treatment of acute brain injury is aided by using bedside cerebral blood flow (CBF) and metabolic monitors for rapid detection of ischemic conditions. Here we demonstrate the cerebral hemodynamic monitoring capabilities of a custom-built non-invasive neurometabolic optical monitor (NNOM) in critically brain-injured adult patients with multimodality (MM) invasive brain monitors (total of 187 monitoring hours across 11 patients). NNOM is a hybrid monitor that interleaves time-resolved near-infrared spectroscopy (TR-NIRS) measurement of regional microvascular oxygen saturation (StO2)[1] with diffuse correlation spectroscopy (DCS) measurement of cortical CBF[2]. In combination, these measurements of CBF and StO2 further provide access to the oxygen metabolic status of the brain[3]. Through exploiting CBF and StO2 information in combination, NNOM successfully distinguished between ischemic conditions and hypermetabolic conditions that arose spontaneously during patient monitoring. NNOM also identified episodes of impaired and intact cerebral autoregulation, and quantified hemodynamic effects from adjustment of pressor drug infusion rates.
METHODS: The protocol was IRB approved and written consent for all subjects was obtained. Eleven patients with subarachnoid hemorrhage (2), traumatic brain injury (4), intracerebral hemorrhage (3), or post-ischemic encephalopathy (2) were enrolled. Prior to enrollment, patients had invasive brain tissue oxygen (PbO2), CBF, intracranial pressure, and microdialysis monitors inserted through a cranial bolt. The NNOM sensor was placed on the forehead adjacent to the bolt. From enrollment to removal of the bolt, approximately 6-8 hours of daily NNOM monitoring was performed concurrently with invasive neuromonitoring. We further combined StO2 and SaO2 measurements to estimate the oxygen extraction fraction (OEF) [3].
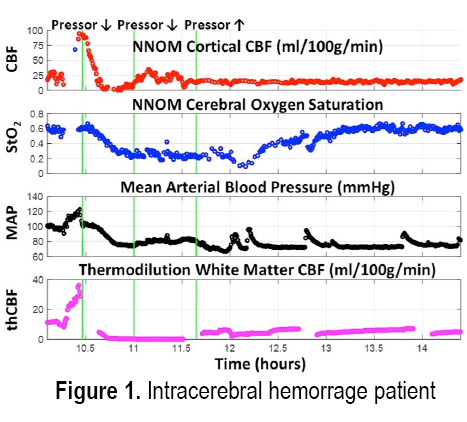
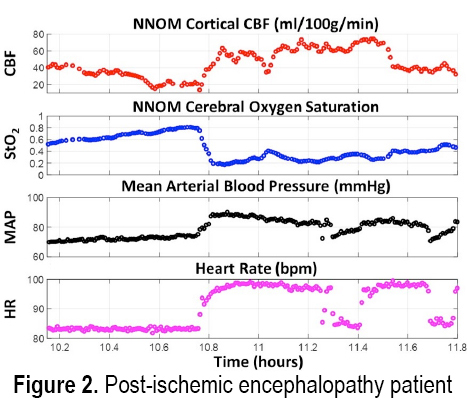
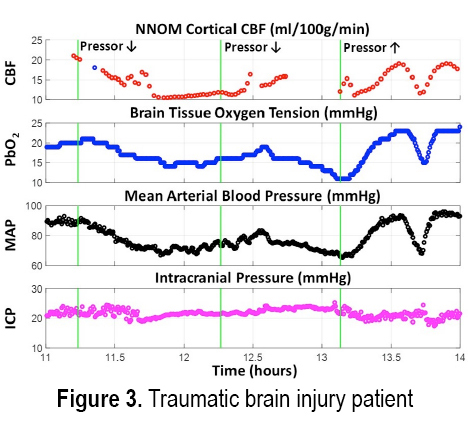
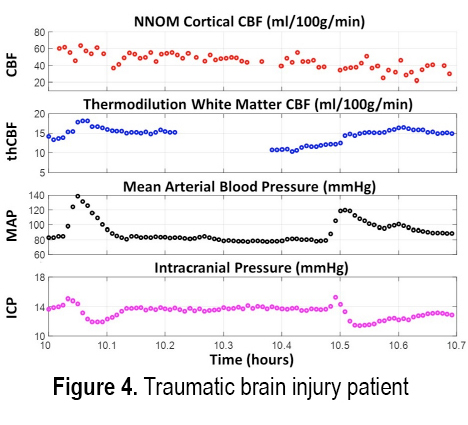
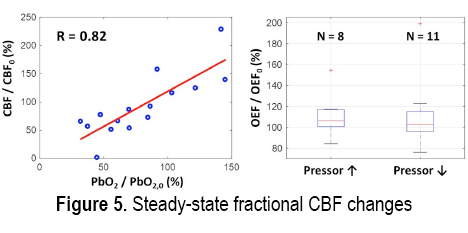
RESULTS: Figures 1 and 2 respectively show examples of ischemic and hypermetabolic conditions (Paroxysmal Sympathetic Hyperactivity, PSH) detected with NNOM. During ischemia, CBF and StO2 decreased together. During PSH, CBF increased, but the larger increase in metabolism resulted in decreased StO2. NNOM further distinguished between impaired (Figure 3) and intact (Figure 4) autoregulation. These NNOM measurements were consistent with invasive measurements. We observed a significant correlation between CBF and PbO2 changes induced by adjustments in pressor medications (Figure 5), but not between OEF and PbO2 changes. In some cases, an increased pressor infusion rate increased OEF, which may reflect metabolic stimulation from pressors crossing an impaired blood-brain barrier.
CONCLUSIONS: We demonstrate the feasibility and potential clinical utility of non-invasive optical monitoring of cerebral hemodynamics.
REFERENCES
1. Neuroimage (2014) 85:28-50, 2014.
2. Neuroimage (2014) 85:51-63, 2014.
3. J. Cereb. Blood Flow Metab. (2003) 23:911-924.
11:25 A.M.
Cerebral hemodynamics improve seizure prediction in multimodal electroencephalographic functional near infrared spectroscopic recordings
Parikshat Sirpal1,2, Ali F. Kassab2, Philippe Pouliot1,3, Dang Khoa Nguyen2, Frederic Lesage1,3
1École Polytechnique de Montréal, Université de Montréal, C.P. 6079, Succ. Centre-ville, Montréal Québec, H3C 3A7, Canada; 2Service de Neurologie, Hôpital Notre-Dame du CHUM, 2099 rue Alexandre-de-Sève, Montréal Québec, H2X 0A93T 1C5, Canada; 3Research Centre, Montreal Hearth Institute, Montreal, Quebec, Canada
In the context of epilepsy, multi-modal approaches have emerged integrating functional near-infrared spectroscopy (fNIRS) with electroencephalography (EEG) to offer dual hemodynamic and electro-potential characterization of a seizure event. Here, we employ novel deep learning methods to investigate the benefits of integrating fNIRS measures for seizure prediction. We designed a deep recurrent neural network with long short term memory units (RNN-LSTM) and used as input multi-modal data from 40 patients between the ages of 22-70 years of age suffering from recalcitrant bilateral temporal lobe epilepsy. Prior consent was obtained and recordings were performed at Notre Dame Hospital, Montréal, Canada and St. Justine Hospital, Montréal, Canada. The multimodal system allowed for approximate full head coverage. For each recording, distinct seizure and non-seizure classes were partitioned. Initially, only EEG data was used as input, followed by fNIRS data and finally multi-modal data. 896 hours of data derived from the CHBMIT scalp EEG database were used for validation purposes of our architecture. Through extensive hyper-parameter optimization and data regularization techniques, we show that multi-modal EEG-fNIRS data provide superior performance metrics in a seizure prediction task with low generalization error and loss, with sensitivity and specificity of 90%, 96% respectively. Either EEG or fNIRS alone led to lower sensitivity and specificity of 82% and 90% and 85% and 92% respectively. False prediction rates were generally low, with 11.84% and 5.61% corresponding to EEG and multimodal data respectively. These results exemplify the enhanced prediction value of multi-modal neuroimaging, particularly fNIRS, in epileptic patients. Furthermore, the neural network models proposed and characterized herein offer a promising framework for future multi-modal EEG-fNIRS investigations in precocious seizure prediction. Furthermore, the value of multimodal recordings will help to confirm seizure prediction and focus localization capabilities of EEG-fNIRS in epileptic patients.
11:40 A.M.
Multifractal hemodynamic fluctuations in the human brain cortex: impact of aging
Peter Mukli1,2, Zoltan Nagy1, Frigyes Samuel Racz2, Peter Herman3, and Andras Eke1,2
1Institute of Clinical Experimental Research, Semmelweis University, 37-47 Tűzoltó Street, Budapest 1094, Hungary; 2Department of Physiology, Semmelweis University, 37-47 Tűzoltó Street, Budapest 1094, Hungary; 3Department of Radiology and Biomedical Imaging, Yale University, New Haven, Connecticut, USA
Fluctuations in resting-state cerebral hemodynamics show scale-free behavior over two distinct scaling ranges. Changes in such bimodal (multi)fractal pattern may reflect altered cerebrovascular or neural function. Our main goal was to assess the distribution of local scale-free properties characterizing cerebral hemodynamics and to unravel the influence of aging on these multifractal parameters. To this end, we obtained extended resting-state records (N=214) of oxyhemoglobin (HbO), deoxyhemoglobin (HbR) and total hemoglobin (HbT) concentration time series with continuous-wave near-infrared spectroscopy technology from the brain cortex. Healthy young (n=24, 30.6±8.2 years) and elderly (n=28, 60.5±12.0 years) volunteers were enrolled in this study. Scaling-range adaptive bimodal multifractal analysis was performed in the time domain yielding generalized Hurst exponent function, H(q), singularity spectrum, D(h) and focus (estimated measure at temporal scale: s=N) separately for a fast and slow component (the latter dominating the highest temporal scales). Endpoint parameters were calculated reflecting degree of long range correlation (Hurst exponent, H(2) and maximal Hölder exponent, hmax and measuring strength of multifractality (full-width-half-maximum of D(h) and ΔH15=H(-15)-H(15)). Correlation-based signal improvement (CBSI) enhanced our signal in terms of interpreting changes due to neural activity or local / systemic hemodynamic influences. We characterized the HbO-HbR relationship with the aid of fractal scale-wise correlation coefficient, r(s) and multifractal covariance analysis. In the majority of subjects, cerebral hemodynamic fluctuations proved bimodal multifractal. In case of slow component of raw HbT, hmax and H(2) were lower in the young group explained by a significantly increased r(s) among elderly at high temporal scales. Regarding the fast component of CBSI-HbT and that of HbO-HbR covariance, hmax and focus was decreased in the elderly group. These observations suggest an age-related progressive deterioration of complex brain neurodynamics reflected by decreased autocorrelation of a neuronal component with an accompanying increased autocorrelation of a non-neuronal component in the aged group.
11:55 A.M.
Real-time color-coded brain cancer mapping for surgical guidance
Hyeon-Cheol Park1, Scott Wu Yuan1, Carmen Kut1, Rachel Sarabia Estrada2, Hugo Guerrero-Cazares2, Sagar R. Shah2, Fausto J. Rodriguez3, Kaisorn Chaichana2, Alfredo Quinones-Hinojosa2, and Xingde Li1
1Department of Biomedical Engineering, Johns Hopkins School of Medicine, Baltimore, MD 21205; 2Department of Neurologic Surgery, Mayo Clinic, Jacksonville, FL 32224; 3Department of Pathology, Johns Hopkins School of Medicine, Baltimore, MD 21205
There are about 80,000 new cases of primary brain tumors each year, which is associated with about 16,000 depth/year. The numbers are much higher when considering non-primary brain cancers. Surgery remains the first line of treatment for brain cancer, and it has been shown that maximal safe resection of the brain cancer can lead to improved overall survival and delayed recurrence. Current intra-operative imaging tools such as surgical microscope, MRI, CT, and ultrasound, have made a considerable impact on guiding brain cancer surgery; however, these modalities have limitations in their ability to provide quantitative, continuous and three-dimensional guidance in real time with the desired resolution, speed, and/or contrast. This presentation will present a newly developed method based on quantitative OCT for providing real time color-coded map of brain cancer during surgery. Our recent studies have shown that the method can distinguish ex vivo human brain cancer from non-cancer with a 92-100% sensitivity and a 80-100% specificity. In this presentation, we will first review the imaging technology, data processing algorithms, imaging protocols, and the detailed results from ex vivo human brain cancer and in vivo mouse brain cancer model imaging studies. In addition, we will present a new and robust algorithm, which is insensitive to the unavoidable imaging field variations during surgery and capable of quantitatively analyzing OCT volumetric data and generating a color-coded optical property map for real-time, continuous guidance in brain cancer resection. Furthermore, we will report our most recent results on intra-operative OCT imaging studies in more than 23 brain cancer patients. Some remaining challenges and our humble experience in translating the technology to clinical practice will be also be shared.
TUESDAY – SESSION III: NOVEL TECHNIQUES AND APPLICATIONS (1)
VIDEOS OF SESSION III
2:15 P.M.
Stimulated Raman scattering microscopy: Seeing the invisible in biology and medicine
Sunney Xie, Ph.D.
Harvard University and Peking University
Stimulated Raman Scattering (SRS) microscopy is a label-free and noninvasive imaging techniqueusing vibration spectroscopy as the contrast mechanism. Highly sensitive, SRS has opened a widerange of biomedical applications, allowing for imaging of small molecules, such as metabolites, drug molecules, or neurotransmitters.
2:55 P.M.
In vivo NAD assay methodology and potential applications for assessing brain NAD+ and NADH contents and redox ratio
Xiao-Hong Zhu, Ph.D.
University of Minnesota Medical School
Nicotinamide adenine dinucleotide (NAD), in oxidized (NAD+) or reduced (NADH) form, can be found in all living cells. As a co-enzyme involved in various redox reactions, NAD plays a key role in regulating mitochondrial ATP production via the NAD+/NADH redox state; as a co-substrate for several important enzymes, NAD+ mediates cellular signaling processes and its level is crucial for cell function and survival. However, measuring the intracellular NAD+ and NADH contents and redox state is very challenging, especially in living human brains. Recently, we developed a 31P MRS-based in vivo NAD assay that enables the quantitative and non-invasive measurement of NAD+ and NADH levels to determine the NAD+/NADH redox ratio in intact organs such as brain. This talk will present a brief overview of the methodology, including the verification of the NAD measurements on brains of different species, the same species at different magnetic fields and under different physiopathological conditions. We will show few examples of potential applications of the NAD assay for studying the alteration of the brain NAD contents and redox state in patients and in healthy human subjects under normal aging. We can also use the same method to evaluate the efficacy of the NAD+ supplementation therapy, which may be able to restore brain NAD+ levels, thereby provide potential treatments for various neurodegenerative diseases or human aging.
3:20 P.M.
mitoRACE: in vivo assessment of mitochondrial function using multiphoton NADH fluorescence
Brad Willingham1, Yingfan Zhang1, and Brian Glancy1,2
1Muscle Energetics Laboratory, NHLBI, NIH, Bethesda, MD 20892; 2NIAMS, NIH, Bethesda, MD 20892
The evaluation of mitochondrial function is critical to the study of energy metabolism in health and disease. However, there are currently limited methodologies that provide direct assessments of mitochondrial oxidative phosphorylation in vivo. Here we describe a novel technique for evaluating mitochondrial function in vivo using reduced nicotinamide adenine dinucleotide (NADH) autofluorescence which provides a direct, spatially-resolved assessment of oxidative phosphorylation.
METHODS: Skeletal muscle redox kinetics were evaluated in vivo by imaging NADH fluorescence in the mouse tibialis anterior muscle using multiphoton microscopy with real-time 3D motion tracking. NADH serves as the primary electron donor of the mitochondrial electron transport chain (ETC). At rest, the mitochondrial NADH pool is the net result of both production by cytosolic dehydrogenases and utilization by the ETC (complex I). To evaluate flux through the ETC, NADH utilization was rapidly inhibited via superfusion of sodium cyanide, resulting in an instantaneous increase in mitochondrial NADH that is proportional to the preceding rate of utilization. Thus, flux through the ETC was quantified as the rate of change in NADH fluorescence immediately following superfusion of sodium cyanide. Mitochondrial flux was assessed in the basal, uncoupled, and fasted metabolic states. Mitochondria were uncoupled via superfusion of 10 µM FCCP, and mitochondrial membrane potential was evaluated using tetramethylrhodamine (TMRM) fluorescence.
RESULTS: At rest, skeletal muscle NADH pools were 75.15±2.97% reduced. Basal mitochondrial flux was 74.58±3.4 µM NADH sec-1. Uncoupling resulted in a complete dissipation of mitochondrial membrane potential and a ~3.3 fold increase in mitochondrial flux (243.3±16.48 µM NADH sec-1). Fasting was associated with a 40% decline in basal flux (45.2±4.79 µM NADH sec-1).
CONCLUSIONS: The Mitochondrial Redox After Cyanide Experiment (mitoRACE) can provide a direct, spatially-resolved assessment of mitochondrial oxidative phosphorylation in vivo with potential to evaluate changes in mitochondrial flux associated with alterations in metabolic status and pathology.
3:35 P.M.
Hyperpolarization chemistry for broadly available metabolic imaging
Thomas Theis, Ph.D.
Duke University, Department of Chemistry, Durham, NC 27708, USA
Over the last decade, hyperpolarized MRI is emerging as an intriguing possibility to observe metabolic transformations and their kinetics in health and disease. Most in vivo demonstrations and current clinical trials use dissolution DNP technology. However, this requires expensive equipment, which costs >$2.5M for the hyperpolarizer and a comparable sum for the superconducting MR scanner. To overcome these issues we develop low-cost alternatives using easy-to-produce parahydrogen gas for hyperpolarization purposes and combine with low-cost, low-field MRI with the goal of obtaining comparable sensitivity at a fraction of the cost.
A further problem of current hyperpolarized MRI experiments is fast decay of hyperpolarization. In good cases lifetimes are about one minute, sometimes less. To prolong signal lifetime, we design spin labeling schemes in small organic molecules that enable long term storage of hyperpolarization. This can be accomplished with spin labels associated with a very long T1 constant or by exploiting long-lived singlet states, which can be obtained on pairs of spins.
We combine low-cost hyperpolarized MRI with long-lived spin states driving towards a next-generation imaging platform that could exceed current approaches in both cost-efficiency and the imaging time window. Specifically, we develop Signal Amplification By Reversible Exchange (SABRE) approaches that use parahydrogen to hyperpolarize 15N nuclei with very long T1, or pairs of 15N nuclei that support long lived singlet states.1,2 Often the hyperpolarization lifetimes (T1 and TS) are particularly long at low magnetic fields of 1 T and below, which can easily be accessed without cryogenically cooled magnets. A variety of hyperpolarized molecular motifs will be discussed that have specific 15N tags including vitamins (e.g. VitB3), drugs (e.g. metronidazole) and metabolites (e.g. choline) and we describe progress in characterizing their relaxation dynamics and chemical turnover at low magnetic fields.3-5 Finally, the intriguing possibility of 15N hyperpolarizing in gas in ortho and long-lived para states will be discussed representing an unexplored quantum reagent.
REFERENCES:
1. T. Theis et al. (2015) J. Am. Chem. Soc. 137:1404-1407.
2. T. Theis et al. (2016) Sci. Adv. 2:e1501438.
3. D. A. Barskiy et al. (2016) J. Am. Chem. Soc. 138:8080-8083.
4. K. Shen et al. (2017) Angew. Chem. Int. Ed. 56:12112-12116.
5. J. Bae, Z. Zhou, T. Theis, W. S. Warren, Q. Wang (2018) Sci. Adv. 4.
TUESDAY – SESSION IV: CANCER (3)
VIDEOS OF SESSION IV
4:35 P.M.
Imaging cancer metabolism in patients: FDG, PET, and beyond
David Mankoff, M.D., Ph.D.
University of Pennsylvania
The identification of altered energy metabolism as a hallmark of cancer [1] leads to opportunities to exploit cancer metabolism as a target for diagnosis. This has been most widely exploited using the elevated rate of glycolysis observed in most tumors as a target for imaging [2]-in specific, positron emission tomography (PET) imaging of the glucose analog 18F-fluordeoxyglucose (FDG). FDG PET, now combined with anatomic imaging in the form of PET/CT, has become an important tool for cancer detection, staging, and response assessment, widely used in oncologic clinical practices around the world [3,4]. Clinical use of FDG PET has increased our appreciation of aberrant glycolysis as a feature of more aggressive, less differentiated tumors [5,6]. At the same time, the variability of tumor FDG uptake seen in clinical imaging has highlighted some of the limitations of imaging tumor glycolysis and has spurred the development of new imaging probes and novel imaging modalities designed to image other facets of cancer energy metabolism. In addition, the use of therapeutics that are targeted to and/or influenced by cancer metabolic phenotype [7] generate drive applications of cancer metabolic imaging to clinical challenges beyond detection that predicting and monitoring response to targeted therapy[8]. This talk will highlight current uses of FDG PET/CT for cancer staging and response evaluation, as well as alternative PET probes targeted to other metabolic substrates such as amino acids, lipids, and TCA intermediates [9].
The talk will also describe the benefit of multi-modality imaging as an approach to fully characterize cancer metabolism, using glutaminolysis imaging as an example of active investigation [10, 11]. Supported in part by Komen SAC130060, DOE DE-SE0012476, NIHP30-CA016520, and NIH R01-CA211337, R33-CA225310.
1. Hanahan, D. and R.A. Weinberg. Hallmarks of cancer: the next generation. Cell (2011) 144:646-674.
2. Gillies, R.J. and R.A. Gatenby. Metabolism and its sequelae in cancer evolution and therapy. Cancer J (2015) 21:88-96.
3. Kelloff, G.J., J.M. Hoffman, B. Johnson, H.I. Scher, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res (2005) 11:2785-2808.
4. Mankoff, D.A., J.F. Eary, J.M. Link, M. Muzi, et al. Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin Cancer Res (2007) 13:3460-3469.
5. Alvarez, J.V., G.K. Belka, T.C. Pan, C.C. Chen, et al. Oncogene pathway activation in mammary tumors dictates FDG-PET uptake. Cancer Res (2014) 74:7583-7598.
6. Palaskas, N., S.M. Larson, N. Schultz, E. Komisopoulou, et al. 18F-fluorodeoxyglucose positron emission tomography marks MYC-over expressing human basal-like breast cancers. Cancer Res (2011) 71:5164-5174.
7. DeBerardinis, R.J., A. Mancuso, E. Daikhin, I. Nissim, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA (2007) 104:19345-19350.
8. Mankoff, D.A., M.D. Farwell, A.S. Clarkand D.A. Pryma. Making molecular imaging a clinical tool for precision oncology: a review. JAMA Oncol (2016)
9. Lewis, D.Y., D. Soloviev and K.M. Brindle. Imaging tumor metabolism using positron emission tomography. Cancer J (2015) 21:129-136.
10. Zhou,R., A.R. Pantel, S. Li, B.P. Lieberman, et al. [(18)F](2S,4R)4 fluoroglutamine PET detects glutamine pool size changes in triple-negative breast cancer in response to glutaminase inhibition. CancerRes (2017) 77:1476-1484.
11. Zhu, L., K. Ploessl, R. Zhou, D. Mankoff, et al. Metabolic imaging of glutamine in cancer. J Nucl Med (2017) 58:533-537.
5:15 P.M.
Fluorescence molecular imaging of cancer metabolism
Samuel Achilefu, Ph.D.
Washington University at St. Louis
5:40 P.M.
Early-time breast cancer oxygen saturation predicts response to neoadjuvant chemotherapy
Arjun Yodh, Ph.D.
University of Pennsylvania
Early-time breast cancer oxygen saturation predicts response to neoadjuvant chemotherapy Abstract Ideally, neoadjuvant chemotherapy (NAC) assessment during therapy should predict pathologic complete response (pCR), a surrogate clinical endpoint for 5-year survival, as early as possible in the course of a typical 3-6 month breast cancer treatment. Herein we introduce and demonstrate a new approach for predicting pCR within 10 days of initiating NAC. The method uses a bedside diffuse optical spectroscopic imaging technology combined with logistic regression modeling. Notably, logistic regression based on z-score normalization using only the tissue oxygen saturation measured within 10 days of the initial therapy dose was found to be a significant predictor of pCR.
JUNE 13, 2018
WEDNESDAY, SESSION I: RADIATION THERAPY AND IMMUNOTHERAPY
VIDEOS OF SESSION I
8:00 A.M.
Reducing cancer hypoxis to improve radiation therapy
Ruth J. Muschel1, Naseer Qayum2, Emmanouil Fokas3, Cat Kelly4, Tom Ashton5, Gillies McKenna1 and Geoffrey Higgins1
1Oxford Institute of Radiation Oncology, Oxford, UK 2Janssen R&D, UK 3Department Radiation Therapy, Frankfurt, Germany 4Perspectum Diagnostics, Oxford, UK 5Adaptimmune, Oxford, UK
Hypoxia, a well recognized hallmark of cancer, facilitates tumor progression and resistance to therapy. Hypoxic cancer cells are resistant to radiation and patients with cancers containing the greatest hypoxia have worse outcomes after radiation therapy than those with less hypoxia. These points lead to the hypothesis that reduction of hypoxia in cancers could render them more susceptible to radiation therapy. Because hypoxia in cancers results from vasculature that is inadequate to supply sufficient oxygen to the tumor, we first asked whether manipulation of the tumor vasculature could reoxygenate tumors. We found that inhibition of the RAS-PI3K oncogenic signaling pathway led to improved vascular function consistent with what is called vascular normalization. As a result tumor oxygenation was improved and response to radiation therapy was greatly augmented.
Since tumor oxygenation is determined both by supply through the vasculature and by consumption, we asked whether reduction in consumption could reduce tumor hypoxia. In fact inhibition of RAS-PI3K signaling reduced oxygen consumption so that using modeling and murine experimetns we could show that approximately 65% of the reduction in hypoxia was due to reduction in oxygen consumption. Taking this further, we performed a screen using FDA approved drugs to identify known pharmaceutical agents that decreased oxygen consumption. One compound so identified, atovaquone an antimalarial drug, was highly effective at reducing oxygen consumption at its therapeutic doses. Atovaquone reduced hypoxia in murine tumors and greatly enhanced the efficacy of radiation therapy. Taken together these data suggest that tumor hypoxia can be subject to pharmacological manipulation to benefit cancer therapy.
8:40 A.M.
Assessing tumor oxygenation and blood flow during photodynamic therapy: applications in treatment personalization
Theresa M. Busch, Ph.D.
University of Pennsylvania
The cytotoxic action of photodynamic therapy (PDT) with many photosensitizers depends on the presence of molecular oxygen. Consequently, tumor physiologic properties such as oxygenation and blood flow can determine the efficacy of treatment. It is well established in animal models that PDT-induced changes in tumor oxygenation and blood flow will correlate with short and long-term treatment outcome. However, few have reported on clinical studies that investigate if physiologic properties of human tumors can associate with PDT outcome. We have investigated the association between lesion oxygenation and PDT outcome in patients treated on a Phase I trial of PDT with aminolevulinc acid (ALA) for high-grade dysplasia, carcinoma-in-situ, or early microinvasive squamous cell carcinoma of the head and neck. Results suggest that measurement of the physiologic properties of previously unresected lesions may play a role in identifying patients with the highest probability of benefiting from PDT. In parallel with clinical investigations, we have undertaken preclinical studies to explore real-time, hemodynamic monitoring of PDT effect on tumor blood flow as a means to inform the delivery of PDT. Relative blood flow was measured in real-time during light delivery for PDT, and through an automated feedback process, it was used to modulate illumination fluence rate so as to conserve tumor blood flow. Long-term tumor response (i.e., time-to-a-400 mm3 tumor volume) was significantly improved by blood-flow informed PDT; moreover, these improvements were not at the cost of lengthening the duration of treatment. Collectively, clinical and preclinical studies support efforts toward developing real-time personalized dosimetry that adjusts illumination parameters for PDT as a function of measured oxygenation and blood flow in the tumor microenvironment.
9:05 A.M.
Radiation therapy mediated molecular imaging
Brian W. Pogue, Ph.D.
Geisel School of Medicine Dartmouth College
A fundamentally new way to sense within tissue was discovered in the past few years, utilizing ionizing radiation to induce optical signals, either through direct Cherenkov generation in the tissue, or through scintillating nanoparticles. This optical signal can be generated by either isotopes or external beam x-ray sources such as keV x-ray tubes or linear accelerators (Linac). Linacs are used to deliver radiotherapy to patients, with photon or electron beams at MegaElectron Volt (MV & MeV) energies, and the electrons resulting from this generate Cherenkov light when passing through tissue. This light emission can be imaged from the surface of patients, and from within the interior. The benefits of both Cherenkov imaging and scintillator for dosimetry will be outlined, and examples of extending the use of this light to sense molecular features of tumors are demonstrated. Radiation-mediated light activation for molecular sensing of oxygen is possible with oxygen sensor PtG4 OxyPhor (University of Pennsylvania), and high resolution mapping of the oxygenation within a tumor is demonstrated recently to near 100 micron spatial resolution. Additionally, receptor density status imaging is also possible using antibody coated scintillating particles, which would report on their uptake in tumor cells. This has been prototyped with EGFR labeled Europoium scintillating nanoparticles. Imaging uptake and pharmacokinetics has been shown and the extension to using these in human tumors is discussed. The benefit of this design for metabolic and immune sensing is that it allows for sampling during fractionated radiotherapy, with only the addition of the molecular sensor to the tumor tissue.
WEDNESDAY – SESSION II: METABOLISM AND FUNCTIONS (3) – ISOTOPE METABOLISM
VIDEOS OF SESSION II
10:15 A.M.
Magnetic resonance spectroscopy studies of the relationship between mitochondrial neuroenergetics and brain function
Douglas L Rothman, Ph.D.
Yale University School of Medicine
Although the brain has been long known to be highly sensitive to alterations in oxidative phosphorylation the energy costs of brain function and the underlying energetic processes have long remained unclear. The difficulty in studying neuroenergetics was partially due to the brain having three primary cell types (excitatory neurons, inhibitory neurons, glia) with specialized metabolic and functional roles. This talk will cover research using magnetic resonance spectroscopy (MRS) and stable isotopes (13C, 15N) that has helped elucidate both the cell type specific energetic cost of brain function as well as key processes that are highly sensitive to energetic impairments in disease states. Among the key findings have been that the majority of brain mitochondrial energy production supports and is linearly proportional to brain signaling and that the two primary neurotransmission pathways (GABA, and glutamate) are supported by metabolic cycles between neurons and astrocytes. The combined flux through these cycles is close to the rate of glucose consumption making excitatory and inhibitory neurotransmission among the highest flux metabolic pathways in the brain. The talk will conclude looking at the implications of these findings for understanding the energetic basis of brain disease and potential therapies.
REFERENCES:
Yuguo Yu, Herman P, Rothman DL, Agarwal D, Hyder F (2017) Evaluating the gray and white matter energy budgets of human brain function. J. Cereb. Blood Flow Metab. Online 6/7/2017.
Abdallah CG, Jiang J, De Feyter HM, Fasula M, Krystal JH, Rothman DL, Mason GF, Sanacora G. (2014) Reduced glutamate metabolism in major depressive disorder. American Journal of Psychiatry (USA). 171 (12):1320-1327
Rothman DL, de Feyter HM, de Graaf RA, Mason GF, Behar KL. (2011) 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR in Biomed. 24(8) Special Issue:943-957.
10:55 A.M.
Metabolic flux analysis studies of cancer cells and tumors by 13C-NMR and 13C LC-MS
A.A. Shestov,1 S-C. Lee,1 K. Nath,1 D.S. Nelson,1 L. Guo,2 I.A. Blair,2 M.A. Wasik,3 D.B. Leeper,4 J.D. Glickson1
1Department of Radiology, 2Department of Systems Pharmacology and Translational Therapeutics,3Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA. 19104, 4Department of Radiation Oncology, Thomas Jefferson University, Philadelphia, PA 19107
Since changes in tumor metabolism often precede changes in tumor volume, monitoring of tumor metabolism may provide a basis for early detection, or perhaps even prediction of therapeutic response. We developed and validated “bonded cumomer analysis” to fit dynamic or steady state 13C MRS data to a metabolic network model that measures flux through key pathways of tumor intermediary metabolism. We have applied this model to studies of human tumor cells in a bioreactor and are currently extending it to in vivo subcutaneous tumors in mice. We validated bonded cumomer analysis by demonstrating agreement between model predicted and experimental rates of oxygen consumption, glucose consumption, lactate production and glutamate pool size. Under aerobic conditions DB-1 melanoma cells, growing as monolayers on microcarrier beads, obtained 49% of their ATP from glycolysis and 51% from oxidative phosphorylation. These cells exhibited low levels of glutaminolysis and high levels of glutamine consumption, reflecting substantial contributions from reductive carboxylation and perhaps other pathways of glutamine metabolism. Using a surface coil, we have measured 13C MRS spectra of subcutaneous tumors in live mice by direct 13C excitation with 1H decoupling at 9.4 T and infusion of [1,6-13C2] glucose. We are applying bonded cumomer flux analysis to this tumor data.
“Fragmented cumomer analysis” has been developed for LC-MS applications and utilized in studies of response of human mantle cell lymphoma (MCL) cell lines to treatment with ibrutinib (IBR), an inhibitor of Bruton’s tyrosine kinase approved by the FDA for treatment of MCL. An IBR-responsive line (MCL-RL) exhibited greater decreases in glycolytic, ox-phos and glutaminolysis flux on treatment with IBR than an IBR-resistant line (MCL-Jeko-1). However, a glutaminase inhibitor (CB-839) exhibited a much greater effect (decreased cell viability) on MCL-Jeko-1 than on MCL-RL even though both lines exhibited similar high levels of glutamine metabolism prior to treatment.
11:20 A.M.
Metabolic imaging of the lungs using hyperpolarized 13C-MRI
Mehrdad Pourfathi, Stephen Kadlecek, Ian Duncan, Maurizio Cereda, Yi Xin, Sarmad Siddiqui, Kai Ruppert, Hooman Hamedani, Harrilla Profka, and Rahim R. Rizi
Department of Radiology, University of Pennsylvania
Several imaging modalities - CT, SPECT, proton MRI and hyperpolarized gas MRI - have been used to examine the structural and functional changes resulting from poor regulation of the lung response to pulmonary inflammation. Though all these approaches have been extremely productive, they examine the secondary effects of inflammation on the lungs rather than targeting the metabolic processes that are the underlying drivers of inflammation-induced changes. Efforts to assess lung inflammation metabolically have previously been undertaken, primarily using position emission tomography (PET) and combined PET/CT to asses abnormalities in the uptake of a glucose analogue (FDG)1,2.
In this presentation, we will discuss hyperpolarized (HP) 13C-MRI’s utility for investigating lung metabolism in animal models that mimic variety inflammatory pulmonary diseases including acute respiratory distress syndrome (ARDS), radiation pneumonitis, and idiopathic pulmonary fibrosis (IPF). We show that HP lactate-to-pyruvate ratio significantly increases in the inflamed lungs, while the arterial lactate concentration only moderately increases. Increased lactate levels in such settings can be associated with infiltration of glycolytically-active neutrophils into the lungs, with systemic or localized tissue hypoxemia which is often concurrent with inflammatory lung injury, or with systemic or localized increases in lactate concentration. To further investigate this, we used HP 13C-MRI to study both lung and overall metabolism in hypoxic animals, and showed an increase in the pulmonary HP lactate-to-pyruvate ratio that was strongly correlated with systemic blood lactate levels under hypoxic conditions. The fact that the HP lactate-to-pyruvate ratio observed in animals with inflamed lungs was larger than can be explained by increased blood lactate levels alone suggests that, in animals with lung inflammatory diseases, increased HP lactate-to-pyruvate ratio is primarily due to the lung inflammation rather than secondary changes in the tissue’s redox state under severe hypoxemia. The potential to expand the utility of HP 13C-MRI to a broader range of pulmonary disorders including lung cancer and pulmonary allograft rejection is a topic I will touch upon here. Lastly, we will discuss the challenges of lung MRI and its translation to larger animal studies and, ultimately, human subjects. we will share our progress on pulse sequence development and demonstrate our preliminary results in porcine studies using the new clinical DNP system.
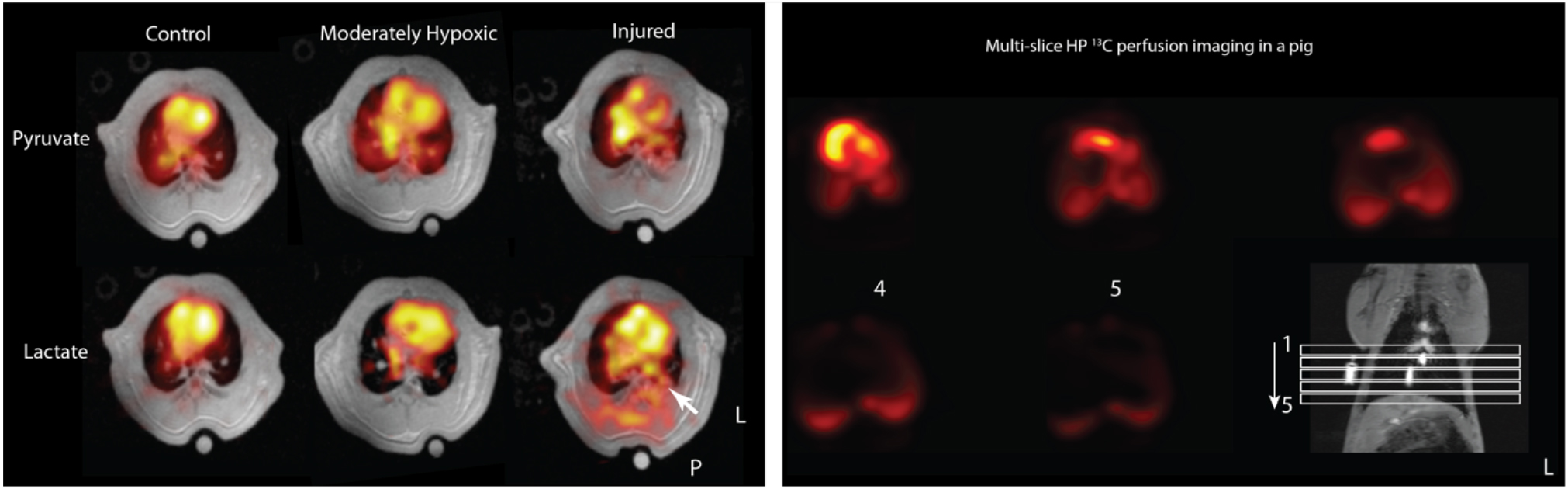
Left: Pyruvate and lactate maps overlaid on T1-weighted axial images in normal, moderately hypoxic (SpO2~90%) and injured rats after 4 hours of mechanical ventilation. We observe a small difference in overall pulmonary lactate levels in the hypoxic rats compared to controls. Lactate signal increased significantly throughout the lung, but most significantly in areas co-localized with consolidation in the lungs. Right: Multi-slice rapid MRI in a pig lung after administration of HP [1-13C] pyruvate.
REFERENCES:
1. Bellani, G. et al. (2011) Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung Injury. Am J Respir Crit Care Med 183:1193–1199.
2. Jones, H.A. et al. (1994) In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med 149:1635–1639.
3. Siddiqui, S. et al. (2016) The use of hyperpolarized carbon-13 magnetic resonance for molecular imaging. Advanced Drug Delivery Reviews doi:10.1016/j.addr.2016.08.011
4. Ardenkjaer-Larsen, J.H. (2003) Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences 100:10158–10163.
5. Nelson, S.J. et al. (2013) Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]-pyruvate. Science Translational Medicine 5 198ra108–198ra108.
6. Cunningham, C.H. et al. (2016) Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res 119(11):1177-1182.
SESSION III: NOVEL TECHNIQUES AND APPLICATION (2)
VIDEOS OF SESSION III
1:35 P.M.
Metabolic imaging of tumor heterogeneity
M.C. Skala1,2, J.T. Sharick1,3, A.J. Walsh1, A.T. Shah3, T.M. Heaster1,2, P. Favreau1, A. Gillette1,2, C. Pasche2, D. Deming2, R.M. Cook3, and C.L. Arteaga3
1Morgridge Institute for Research, Madison, WI; 2 University of Wisconsin, Madison, WI; 3Vanderbilt University, Nashville, TN
Abnormal cellular metabolism is a hallmark of many diseases, yet there is an absence of quantitative methods to dynamically image metabolism with cellular-level resolution. Optical metabolic imaging (OMI) quantifies the fluorescence intensities and lifetimes of the metabolic co-enzymes NAD(P)H and FAD using two-photon microscopy1-8. OMI is a label-free, high-resolution, quantitative tool for monitoring cellular metabolism within intact samples. In vivo OMI is sensitive to heterogeneous changes in cellular metabolism induced by clinically relevant anti-cancer therapies in mouse models of cancer. The ability to monitor these treatment-resistant sub-populations of cells in vivo is important for identifying and eliminating the particularly lethal cells that cause tumor recurrence and metastases. We have further developed a “tumor-in-a-dish” organoid platform to rapidly test multi-drug response using OMI. This platform has been validated in mouse models of breast and pancreas cancer, and feasibility has been tested in human tumors with chemotherapies, targeted therapies, and experimental drugs. Importantly, the cellular-level assessment of OMI allows for sub-populations of cells with varying response to drug treatment to be tracked over time, to achieve therapeutic effect in all cell populations. This attractive suite of metabolic imaging tools has significant implications for rapid cellular-level assessment of metabolic response to drug treatment (1) in vivo, (2) in high-throughput drug efficacy studies, and (3) as a clinical tool to plan individualized treatment regimens. Therefore, these technologies could greatly accelerate cures for cancer patients.
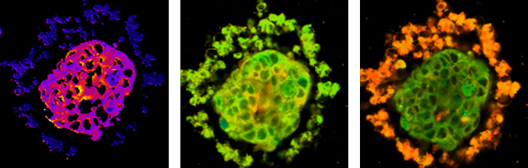
Figure 1: Optical metabolic imaging of breast cancer organoid.
Left - redox image, middle – NAD(P)H fluorescence lifetime image, right – FAD fluorescence lifetime image.
REFERENCES:
1. Walsh, A.J., Cook, R.S. and Skala, M.C. (2017) Journal of Nuclear Medicine jnumed-117.
2. Shah, A.T., T.M. Cannon, J.N. Higginbotham, R.J. Coffey, and M.C. Skala (2017) J Biophotonics 10(8):1026-1033.
3. Shah, A.T., K.E. Diggins, A.J. Walsh, J.M. Irish, and M.C. Skala (2015) Neoplasia 17(12):862-870.
4. Walsh, A.J., J.A. Castellanos, N.S. Nagathihalli, N.B. Merchant, and M.C. Skala (2015) Pancreas
5. Walsh, A.J., R.S. Cook, H.C. Manning, D.J. Hicks, A. Lafontant, C.L. Arteaga, and M.C. Skala. (2013) Cancer Res 73(20):6164-6174.
6. Walsh, A.J., R.S. Cook, M.E. Sanders, C.L. Arteaga, and M.C. Skala (2016) Sci Rep 6:18889.
7. Walsh, A.J., R.S. Cook, M.E. Sanders, L. Aurisicchio, G. Ciliberto, C.L. Arteaga, and M.C. Skala (2014) Cancer Res 74(18):5184-94.
8. Walsh, A.J. and M.C. Skala. (2015) Biomed Opt Express 6(2):559-573.
9. Shah, A.T., T.M. Heaster, and M.C. Skala (2017) PLoS One 12(1):e0170415.
2:00 P.M.
Translating optical molecular imaging
Jim Delikatny, Ph.D.
University of Pennsylvania
We describe the development and implementation of fluorescent probes for assessment of tumor metabolism using optical imaging techniques. Near-infrared optical imaging is a powerful and transformative technology that allows assessment of biological function in vivo. This ability is provided through exogenous fluorescent or luminescent contrast agents that report directly on enzyme activity or expression, or on local tumor environment. These compounds can be created via conjugates to molecules of biological interest or through attachment to a backbone structure that allows activation through cleavage or rearrangement. A major focus of our lab has been on fluorescent probes that report on lipid metabolism and tumor microenvironment. We have developed probes for detection of tumor microenvironment pH using Cerenkov imaging1, activatable probes for detection of phospholipases2 and probes for detection of choline kinase (ChoK)3,4. Cerenkov probes detect the light emitted by beta-decaying radioisotopes. When attached to an optical sensor, such as a pH indicator, the change in light emission resulting from pH changes can be detected in vivo. This can be combined with quantitative information provided by PET to give both functional and anatomic information. The phospholipase probes are based on a caged design in which a fluorophore is esterified with arachidonic acid to impart specificity to cPLA2, the signaling phospholipase at the top of the prostaglandin signaling cascade important for tumor growth and progression. These probes report on cPLA2 expression in tumor models and can distinguish breast cancer sub-types in vitro and in vivo. The ChoK sensors are based on a unique design in which an NIR fluorophore is modified to contain a mimetic group that interacts directly with the enzyme active site and functions as a competitive inhibitor. These probes report on ChoK expression in breast and lung tumor models and can monitor the response to anticancer therapy in vivo. Our current efforts focus on translation of the ChoK probes for the detection of tumor margins in an intraoperative setting5. Here we are developing a large animal clinical trial to detect tumor margins and guide surgical resection in spontaneous lung tumors in patient dogs.
1. Czupryna, J., Kachur, A.V., Blankemeyer, E., Popov A.V., Karp, J.S and Delikatny, E.J. (2015). In vivo pH detection using switchable 18F labeled Cerenkov probes. J Nucl Med. 56:483-488.
2. Chiorazzo M., Bloch N., Popov A.V. and Delikatny E.J. (2015) Synthesis and evaluation of cytosolic phospholipase A2 activatable fluorophores for cancer imaging. Bioconjugate Chemistry 26:2360-2370.
3. Arlauckas, S.P., Kumar, M., Popov, A.V., Poptani, H. and Delikatny, E.J. (2017) Near infrared fluorescent imaging of choline kinase alpha expression and inhibition in breast tumors. Oncotarget 8:16518-16530.
4. Arlauckas, S.P., Popov, A.V. and Delikatny, E.J. (2016) Choline kinase alpha–Putting the ChoK–hold on tumor metabolism. Prog Lipid Res. 63:28-40.
5. Judy, R., Keating, J., DeJesus, E., Jiang, J., Okusanya, O., Nie, S., Holt, D., Arlauckas, S., Low, P., Delikatny, E.J. and Singhal, S. (2015) Quantification of tumor fluorescence during Intraoperative optical cancer imaging. Scientific Reports 5:16208.
2:25 P.M.
Real-time time-resolved fluorescence lifetime measurements and portable point-of-care diagnostic system for bloodborne pathogens
L. Powers, J Fairbanks, D. Todd, M. Nadolny, P. Hall, J. Roveda, and W. Ellis, Jr.
University of Arizona
Fluorescence technologies have been used to measure lifetime-based signals for many years, and recently these have been reported in tissue. However, real-time measurements can also track the production of reactive species (e.g. ozone) and other intermediate/short-lived states in chemical reactions in the atmosphere as well as metabolic reactions in cells and tissue. We have developed a high speed fluorescence prototype instrument capable of measuring fluorescence lifetimes in real-time using high intensity LED pulses and novel PMT gating technology. Precision timing circuitry generates tunable width pulse signals which are driven through the LED using a comparator-based push-pull architecture. The timing circuitry also generates PMT gating pulses which are applied to the dynode chain via high voltage operational amplifiers. LED pulses with fall times (99%) as short as 2ns and PMT gating times (10% to 90%) of 3.6ns have been achieved. The prototype has been used to measure the fluorescent lifetimes of dyes with and without binding to biological molecules as well as metabolites in live bacterial cells.
2:50 P.M.
Total-body metabolism, chemistry and physiology: PET for fetus to adults
Thomas Budinger, M.D., Ph.D.1, Terry Jones2 , Qiyu Peng1 , Siwei Xie3
1University of California, Berkeley; 2 University of California, Davis; 3Huazhong University of Science and Technology, China
The principal advantages of radiotracer imaging using radionuclides such as carbon-11, oxygen-15, nitrogen-13 and fluorine-18, that decay by positron emission, are the ability to quantitatively study regional specific human biochemistry and physiology with orders of magnitude more sensitivity than magnetic resonance imaging and spectroscopic techniques. This enables exploitation of the true tracer principal The disadvantages are the requirement for administration of radiotracers and more importantly, the fact that only the concentration, and time-activity of the radioactive atom is detected and not the chemical identity of the compound being investigated. Nevertheless, because the affinities of the biochemical receptor and pathway targeted by the injected compound are high, using established physiological kinetic models, reliable inferences regarding the interpretation of the temporal changes in detected activity from regions of the body can be made with confidence. The radiation dose to the human subject can be limiting, yet there is a solution for this disadvantage by instrumentation innovations that can give almost a 20-fold increase in sensitivity for high spatial resolution imaging and more if just whole organ data are required. The reduction in radiation dose enables safe studies of the fetus as absorbed doses can be less than those of a few days of natural background (Jones and Budinger 2013}.
A disadvantage of both radionuclide techniques and magnetic resonance methods is that current instruments do not allow simultaneous observations of the total-body metabolism due to their limited axial extent. The latter can be solved with more economical detectors for radioisotope dynamic imaging studies and superconductor materials advances and extended RF coil designs for magnetic instruments.
This paper presents the medical science rational and potentials for total-body positron emission tomography that has been under development at a few institutions over the past 5 years (e.g. Cherry et al. 2018). The first 2-meters long total-body PET has now been built, and following physical testing will be ready to undertake human studies.
3:15 P.M.
Early diagnosis of traumatic intracranial hematomas
Baruch Ben Dor, PhD1,2, Hasan Ayaz, PhD1, Meltem Izzetoglu, PhD1, Kurtulus Izzetoglu, PhD1, Banu Onaral, PhD1
1Drexel University, Philadelphia, PA; 2InfraScan, Inc., Philadelphia, PA
BACKGROUND: Timing of the intervention for intracranial hematomas is critical for good outcomes, specifically since expansion of the hemorrhage can result in debilitating, and sometimes, fatal outcomes. Led by Britton Chance, a team from University of Pennsylvania, Baylor and Drexel Universities developed a handheld brain hematoma detector for early triage and diagnosis of head trauma victims. The project was supported initially by the Office of Naval Research and later by the Marine Corps System Command. After obtaining De Novo FDA clearance, over two hundred systems were deployed in all Marine battalion aid stations around the world.
METHODS: The Infrascanner, a handheld brain hematoma detection system, is based on the differential near infrared light absorption of the injured vs. the non-injured part of brain. Eleven studies have been conducted with over 1,200 patients having 195 hematomas. The studies were performed in the USA, Canada, Spain, Italy, Nederlands, Russia, Poland, India, China and Turkey.
RESULTS: Six of the studies included adults of all ages and five studies were dedicated pediatric studies for children less than 18 years old. Patients were eligible for the studies if they were undergoing a CT scan within 12 hours of a blunt or penetrating head injury. Infrascanner demonstrated high sensitivity (92%) and specificity (88%) in detecting intracranial hematomas larger than 3.5 mL in volume, and less than 2.5 cm from the surface of the brain. The negative predictive value was 99%.
CONCLUSION: The Infrascanner is a clinically effective screening solution for head trauma patients in pre-hospital settings where timely triage is critical. It is intended to be used as an adjunct to the standard diagnostic workup to aid in the decision of choosing evacuation destination (a trauma center or the nearest hospital) and in prioritizing patients with suspected hematomas for urgent CT scans and surgical interventions.
SESSION IV: NOVEL TECHNIQUES AND APPLICATION (3)
VIDEO OF SESSION IV
4:00 P.M.
Temporal metabolic partitioning of the yeast cellular network: the cell is a global scale-invariant (fractal or self-similar), multi-oscillator
David Lloyd, D.Sc., Ph.D.
School of Biosciences, Cardiff U., Wales, UK
The overall temporal organization of mass-energy, information, and signalling networks exhibited by yeast as a model unicellular eukaryote in self-synchronized continuous cultures represents, until now, the best characterized example of in vivo de-convolution of the time structure of a living system.
Continuous online monitoring (O2 electrode and membrane-inlet mass spectrometry for O2, CO2, and H2 S; and direct measurement of NAD(P)H and flavin fluorescence) in continuous cultures of Saccharomyces cerevisiae IFO-0233 (Inst. of Fermentation, Osaka, Japan) gives strain specific dynamic information from timescales of minutes to hours. Complemented with MS-based metabolomics and transcriptomics, the predominantly oscillatory behavior of network components becomes evident, where the spontaneously synchronized cellular respiration cycles between discrete periods of increased oxygen consumption (oxidative phase) and decreased oxygen consumption (reductive phase). This temperature-compensated ultradian clock provides a coordinating function linking mitochondrial energetics and redox balance to transcriptional regulation, mitochondrial structure and organelle remodeling, DNA duplication, and putatively to chromatin dynamics. Anabolism and catabolism thus become globally partitioned: mediation is by direct feedback loops between the energetic and redox state of the cell and its growing ultrastructural development.Multi-oscillatory outputs occurred in dissolved gases with 12-h, 40-min and 4-min periods, and statistical self-similarity in Power Spectral and Relative Dispersional analyses: i.e. complex non-linear behavior and a functional scale-free network operating simultaneously on several timescales. Fast sampling (at 100 or 1 Hz) of NAD(P)H fluorescence revealed signals at 40 and 3–5 min. The latter corresponds to oscillations directly imaged by 2-photon microscopy in surface-attached organisms. Signaling between time domains is confirmed by studies with protonophore effectors of mitochondrial energetics. Multi-oscillatory states impinge on the complex reactome (where concentrations of most chemical species oscillate) and network functionality is evident as a self-similar system performing over at least 4 orders of magnitude of in vivo time structure measured in seconds.
4:25 P.M.
Engineering freedom of cofactor selection in de novo protein design
Christopher Moser, Ph.D.
University of Pennsylvania
De novo design of cofactor containing proteins provides engineering freedom exploited in two main efforts. First, they allow us to critically test our understanding of the principles of function of natural proteins in alternative frameworks that are adaptable and structurally transparent. This follows Richard Feynman’s adage “What I cannot create, I do not understand”. Second, they allow us to engineer new proteins with no sequence resemblance to any natural protein to perform new or improved functions not found in Nature. Our de novo designs assemble a wide range of light and redox activated cofactors in alpha-helical bundle frameworks for functions ranging from light-activated charge separation and catalysis in artificial photosynthetic reaction center proteins to magnetic field sensing proteins to oxygen transport in artificial hemoglobins.
4:50 P.M.
Mesoscopic functional imaging with fluorescence laminar optical tomography
Yu Chen, Ph.D.
University of Maryland at College Park
5:15 P.M.
Conversion of a fluorescent probe to a PET radiotracer: application in the development of CNS probes
Robert H. Mach
University of Pennsylvania
Positron Emission Tomography (PET) is a powerful in vivo imaging technique that enables the study of the molecular basis of disease. Although significant advances have been made in the instrumentation used in translational PET imaging studies (e.g., PET/CT, PET/MR), a major limitation of the utility of this technique in translational imaging studies is the development of PET radiotracers for imaging specific biological processes. A key step in this process is the identification of a suitable lead compound for PET radiotracer development, and the subsequent structure-activity relationship studies needed to convert the lead compound into a PET radiotracer. The focus of this talk will be on the use of fluorescent probes that have been used in microscopy or optical imaging studies as lead compounds for PET radiotracer development, and the application of these probes to study different mechanisms believed to play a major role in neurodegeneration.
