How does molecular dysfunction drive synaptic, circuit and behavioral dysregulation?
Mice can be powerful animal model to study brain diseases with strong genetic components such as autism, obsessive-compulsive disorder (OCD), Tourette Syndrome (TS) attention-deficit-hyperactivity disorder (ADHD) and intellectual disability (ID). Our lab uses genetic mouse models to reveal convergent changes to the specific neural circuits that drive maladaptive behaviors.
Circuit-Behavioral Analyses in Mouse Models: Finding and Correcting Disease-Associated Neural Signals
Our approach focuses on trans-diagnostic behavioral functions that are central to neurodevelopment disorders including appetitive and social reward processing (autism, ID), spontaneous motor output (autism, TS), sensory-motor processing (ADHD, autism, TS), negative reinforcement (OCD), and completion monitoring (OCD).

(A, left) Image from operant nose-poke task (volume comparisons shown in inset) where mice poke to get chocolate reward. (A, right) Neurexin1α KO mice, but not heterozygote mice, have deficits in returning to larger volume outcomes across a range of volume contrasts. (B, left) Image from social-operant chamber where mice nose-poke to interact with a 'social partner.' (B, right) 1-p Miniscope calcium imaging in mPFC neurons during this task to see how neural representations of social reward are altered in genetic autism models. (C, left) Investigating stereotyped spontaneous movement in Zswim6 mutant mice using DeepLabCut and B-SOiD behavioral classifier. (C, right). The sequence of behavioral fragments can be analyzed to quantify the degree and patterns of motor stereotypy. Zswim6 KO from dSPNs results in an increase in the number of motor fragment transitions per session.
Relevant Papers: Ferrigno et al., bioRxiv 2025; Choi et al., Science Advances 2025; Xu et al., PLos Genetics 2023; Alabi et al., eLife, 2020
Molecular Targets: Understanding Biology for Translational Amelioration
Ground-breaking progress in the genetics of autism and ID, together with recent successes for OCD, ADHD and TS, has highlighted two recurrent molecular pathways - genes encoding synaptic proteins and epigenetic regulators. Our lab has made several important discoveries regarding the role of Neurexin1α, a presynaptically localized molecule associated with autism, schizophrenia, TS and ADHD, within cortico-striatal circuits. More recently, we have focused on the contributions of epigenetic dysregulation to neural circuit function. Working together with the labs of Zhaolan Zhou and Erica Korb, we are investigating the unique epigenetic requirements of neurons that render them vulnerable to genetic perturbations of chromatin regulators. For both synaptic and epigenetic dysfunction, we are particularly interested in the plasticity of these systems in the adult - can genetic or pharmacological therapies be effective after circuits have undergone maturation?
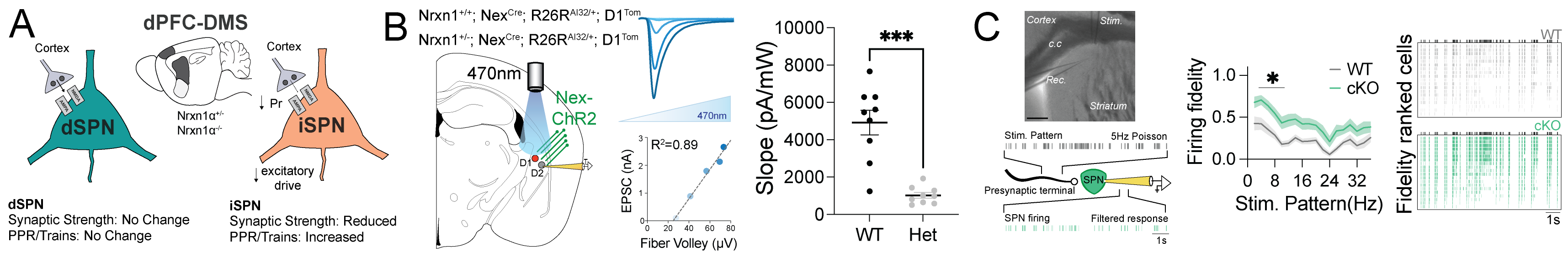
(A) PFC-dorsomedial striatal synapses onto iSPNs are specifically altered in Nrxn1α het and KO mice. (B) Optogenetic measures of cortico-striatal strength in the tail of striatum show that the impaired iSPN recruitment is present throughout the striatum of Nrxn1 mutant mice. (C) Zswim6 disruption in SPNs causes a cell-autonomous increase in cortical recruitment.
Relevant Papers: Ferrigno et al., bioRxiv 2025; Choi et al., Science Advances 2025; Davatolhagh et al., Cell Reports 2021; Terzic et al., Journal of Clinical Investigation 2021; Tischfield et al., Neurobiology of Disease 2017

