Translational Research
Leveraging immunometabolism in enhancing immunotherapy
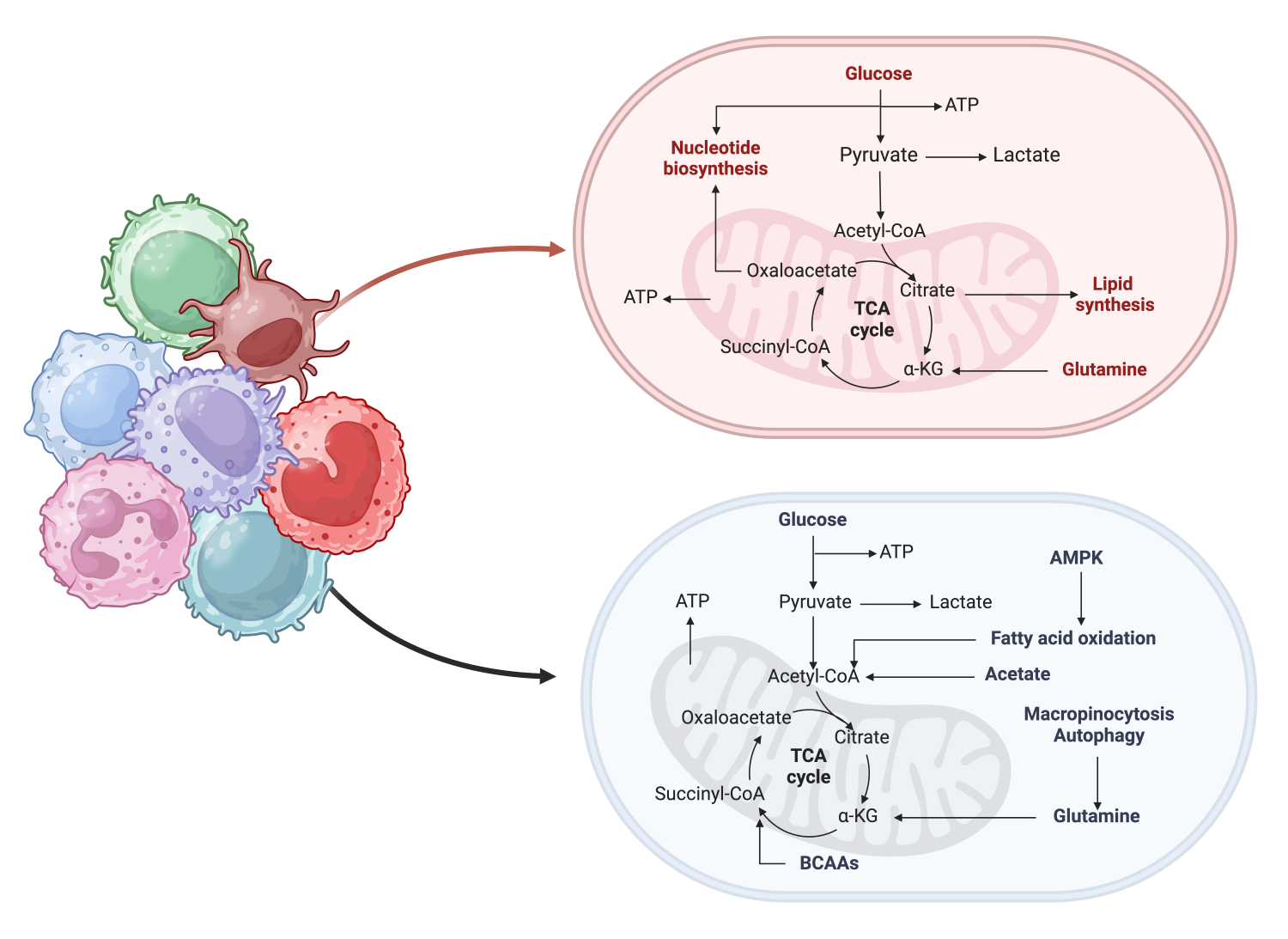
Emerging evidence suggests that metabolic reprogramming of immune cells plays an important role in their development and functional states including alterations of anti-tumor immune responses to immunosuppressive and pro-tumoral responses. Glioblastoma carries a poor prognosis with a median survival of less than 15 months despite surgical resection and chemoradiation. One key component that has limited the efficacy of immunotherapy in glioblastoma is the significant immunosuppressive tumor microenvironment. We aim to determine how metabolic networks regulate immune cell function within the tumor microenvironment to identify novel immune-targeted therapies.
Characterization of the dynamics of tumor infiltrating T cells and response to immunotherapy
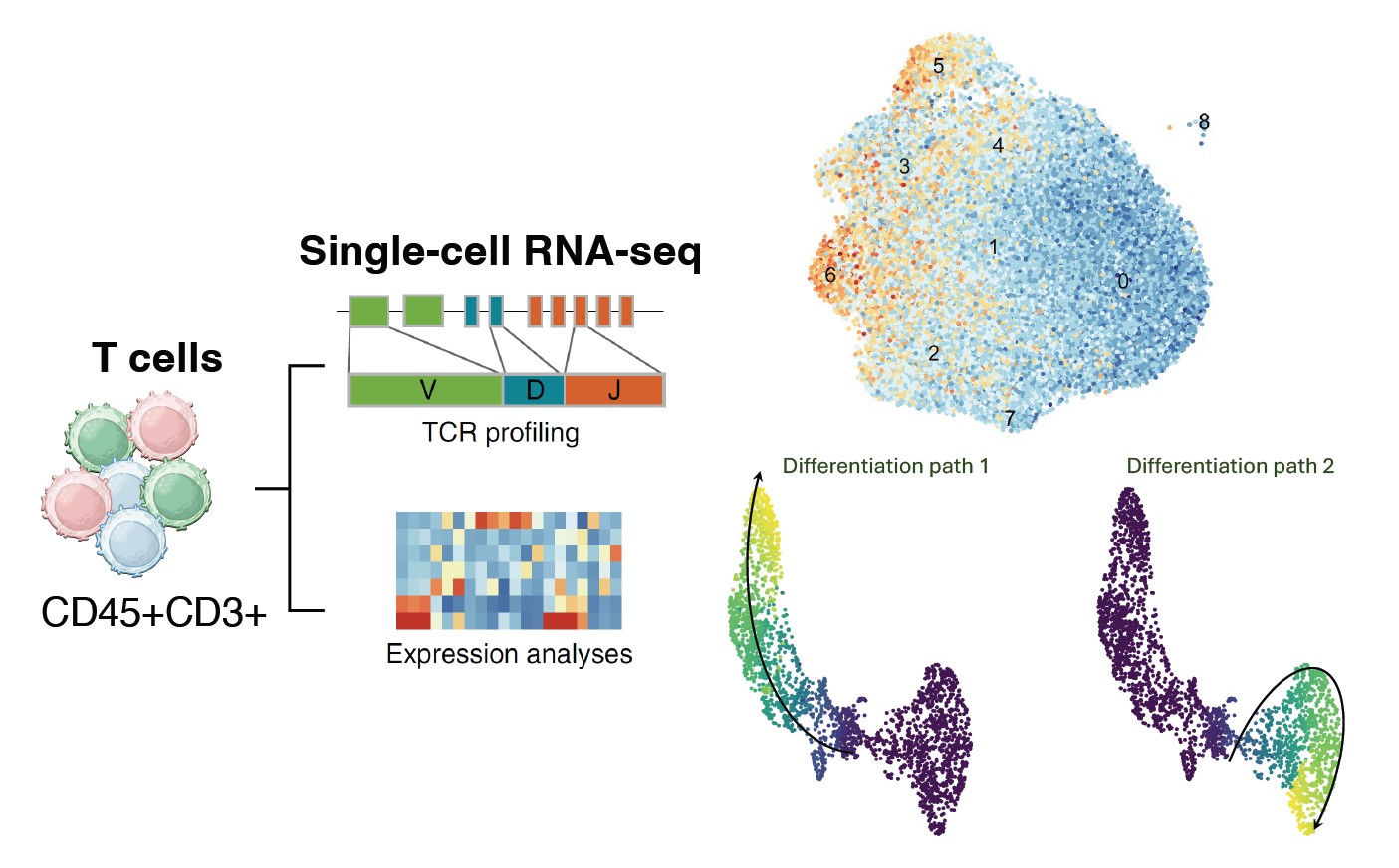
In tumors, T cells can develop a dysfunctional state due to persistent exposure to tumor antigens and immunosuppressive signals within the tumor microenvironment, characterized by diminished effector functions. This phenomena is especially prominent in brain tumors. By leveraging advanced single-cell multiomic and TCR sequencing technologies, we aim to elucidate the mechanisms of tumor-specific T cell exhaustion and clonal lineage of dysfunctional T cells. Our goal is to identify novel therapeutic targets that can rejuvenate exhausted T cells and enhance the effectiveness of immunotherapy for brain tumor patients.
Unraveling immunological drivers of meningioma high-grade progression through integrative single-cell and spatialomics
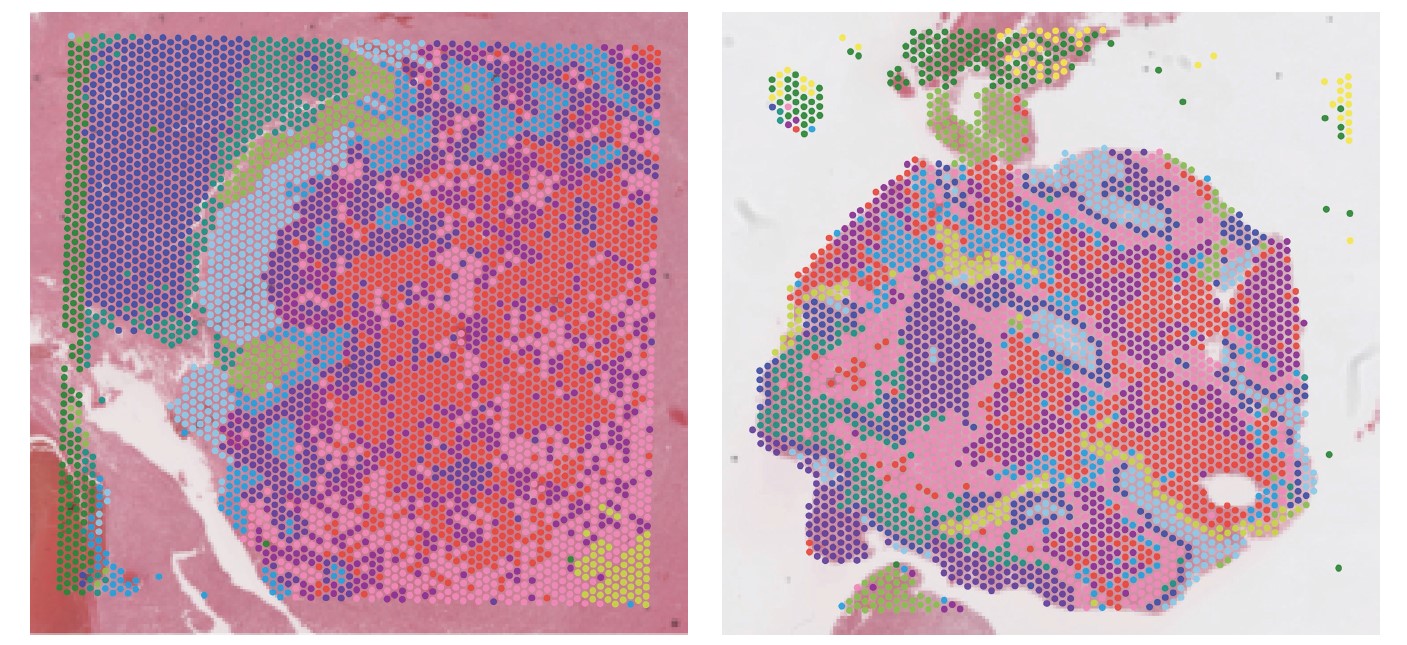
Meningiomas, the most prevalent primary brain tumors, exhibit varying degrees of aggressiveness, with high-grade tumors posing significant challenges due to their propensity for recurrence and invasion into the surrounding brain. There are currently limited treatment options for patients with recurrent high-grade meningiomas. The tumor immune microenvironment plays a crucial role in tumor recurrence and progression, yet its specific contributions in meningioma progression remain poorly understood. We aim to investigate the immunological impact underlying meningioma progression and invasion using innovative multiomic and spatialomic techniques.

