Research
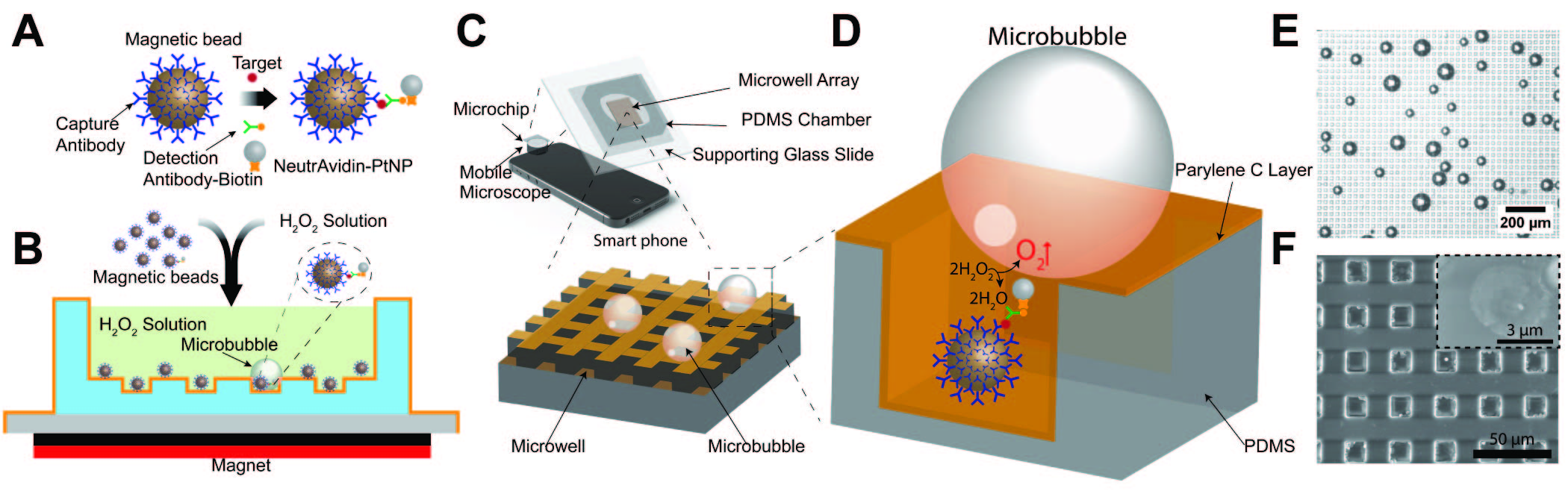
Microbubbling Digital Assay
Labeling target proteins with microbubbles enables highly sensitive (femtomolar) detection of protein biomarkers in a digital assay at the point-of-care (POC). The microbubbling readout can be detected using a smartphone camera and analyzed using an application for automated image analysis developed using machine learning. The nanoparticle-catalyzed microbubbles bridge the “nano” world to the “micro” world with a novel signal modality.
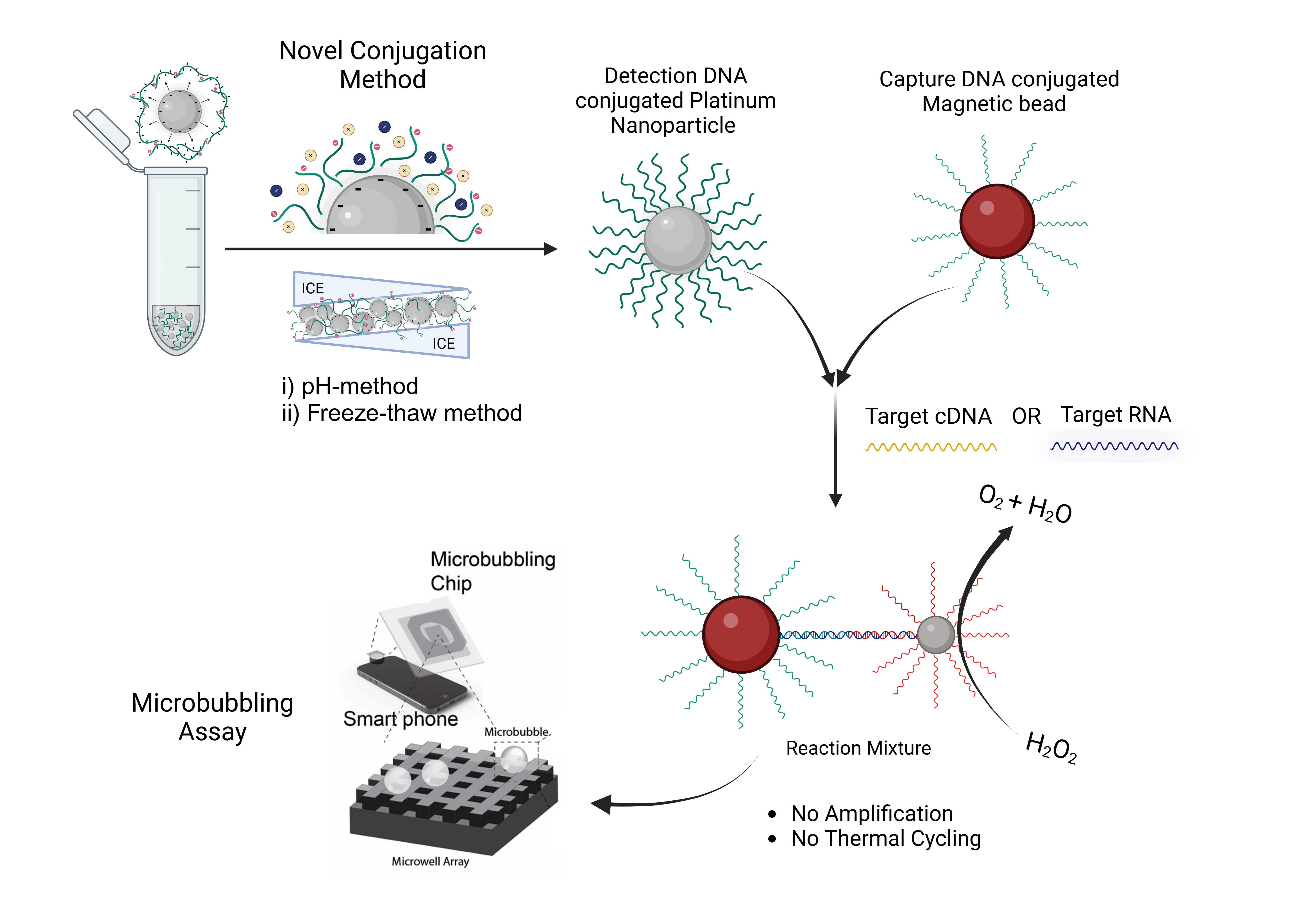
Direct Nucleic Acid Quantification with the Microbubbling Digital Assay
Although significant research exists for synthesizing DNA-gold nanoparticle (AuNP) conjugates, methods for synthesizing stable nucleic acid-PtNP conjugates, especially for larger PtNPs (>50 nm), remain unreported. The instability and slow ligand exchange rate of PtNP colloidal solutions make this synthesis challenging, as conventional methods such as salt aging cause PtNP aggregation and require prolonged durations. We developed a novel DNA-PtNP conjugation method featuring a 30 min pH-mediated conjugation followed by a 30 min freezing at −20 °C. This conjugation approach is rapid, efficient, and sonication-free, resulting in high DNA loading and hybridization efficiency and stable conjugates. Using these conjugates in the microbubbling digital assay enables the assay to detect and quantify nucleic acids in the attomolar range.
Wearable Devices for Automated Drug Delivery
In collaboration with Penn Engineering, we are actively developing autonomous systems for reagent sensing and automated drug delivery. These integrated, dynamic devices will allow for rapid delivery of life-saving medication for drug overdose prevention.

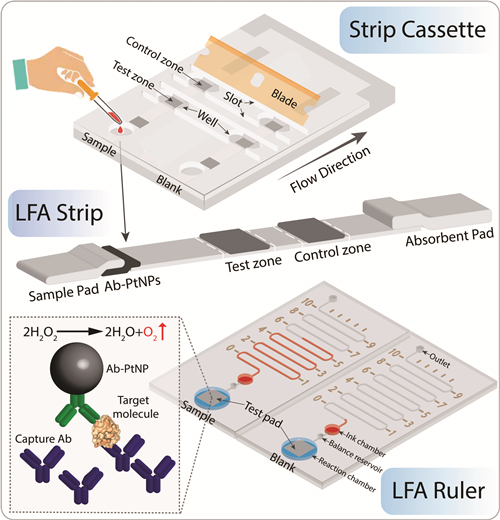
Lateral Flow Assay Ruler
Lateral flow assay (LFA) is a well-established platform for point-of-care (POC) testing due to its low cost and user friendliness. Conventional LFAs provide qualitative or semi-quantitative results, and require dedicated instruments for quantitative detection. Here we developed an “LFA Ruler” for quantitative and rapid readout of LFA results, using a 3D printed strip cassette and a simple, inexpensive microfluidic chip. Platinum nanoparticles are used as signal amplification reporters, which catalyze the generation of oxygen to push ink advancement in the microfluidic channel. The concentration of target is linearly correlated with the ink advancement distance. The entire assay can be completed within 30 minutes without external instrument and complicated operation. We demonstrated quantitative prostate specific antigen testing using LFA ruler, with a limit of detection of 0.54 ng/mL, linear range 0-12 ng/mL, and high correlation with the clinical, gold standard assay. The LFA ruler achieves low cost, quantitative, sensitive and rapid detection, which has great potential in POC testing and can be extended to quantify other disease biomarkers.
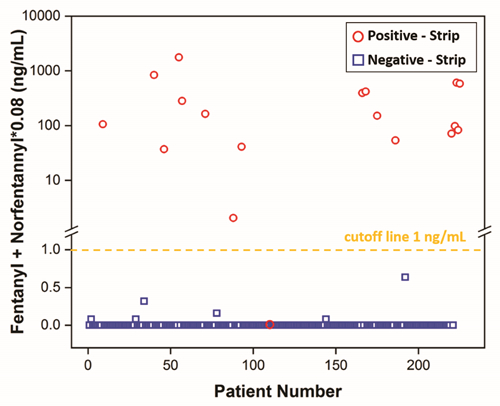
Rapid Fentanyl Screening LFA Strip
Rapid identification of fentanyl at the point of care (POC) is critical. Urine fentanyl concentrations in overdose cases start at single-digit ng/mL. No fentanyl POC assay with cutoff at single-digit ng/mL is available. A competitive lateral flow assay (LFA) was developed using gold nanoparticles and optimized for rapid screening of fentanyl in 10 minutes. Urine samples from two cohorts of Emergency Department (ED) patients were tested using the LFA and a liquid chromatography tandem mass spectrometry (LC-MS/MS) method. The two cohorts consisted of 218 consecutive ED patients with urine drug-of-abuse screen orders, and 7 ED patients with clinically suspected fentanyl overdose, respectively. The LFA detected fentanyl (≥1 ng/mL) and major metabolite norfentanyl (≥10 ng/mL) with high precision. The assay demonstrated no cross-reactivity with amphetamine, cocaine, morphine, tetrahydrocannabinol, methadone, buprenorphine, naloxone and acetaminophen at 1000 ng/mL, and had 0.03%, 0.4% and 0.05% cross-reactivity with carfentanil, risperidone and 9-hydroxyrisperidone, respectively. In 218 consecutive ED patients, the prevalence of cases with fentanyl ≥1 ng/mL or norfentanyl ≥10 ng/mL was 5.5%. The clinical sensitivity and specificity (95% confidence interval (CI)) of the LFA were 100% (75.8-100%) and 99.5% (97.3-99.9%), respectively. The positive and negative predictive values (95% CI) were 92.3% (66.7-98.6%) and 100% (98.2-100%), respectively. The concordance between the LFA and LC-MS/MS was 100% in the 7 suspected fentanyl overdose cases (5 positive, 2 negative). The LFA is able to detect fentanyl and norfentanyl with high clinical sensitivity and specificity in the ED population with rapid fentanyl screening needs.