GTMS Requirements
The Graduate Training in Medical Science (GTMS) Certificate Program complements our existing doctoral programs in Biomedical Graduate Studies. The GTMS program was specifically designed for students who wish to pursue research training at the interface of basic science and medicine. The GTMS program augments graduate training by offering courses that bridge basic and medical science and provides a foundation in the molecular basis of disease.
The GTMS program consists of 4 courses (two that fulfill both graduate program and GTMS requirements and two that emphasize the integration between medicine and basic science), a clinical clerkship and a capstone requirement (paper and presentation at the GTMS Seminar series).
The GTMS curriculum begins in the first or second year of graduate school when you develop a curricular plan with GTMS mentors that includes two electives that fulfill your specific graduate program requirements and also directly relate to your interests in translational research. During your second year, you also take the Molecular Basis of Disease (BIOM 5020), a course designed for GTMS students that explicitly connects disease mechanisms to disease presentation and treatment. Finally, you are also required to take at least one course from a suite of more explicitly “Bench to Bedside”, graduate level elective offerings that emphasize biomedical topics and are designed for interprofessional audiences.
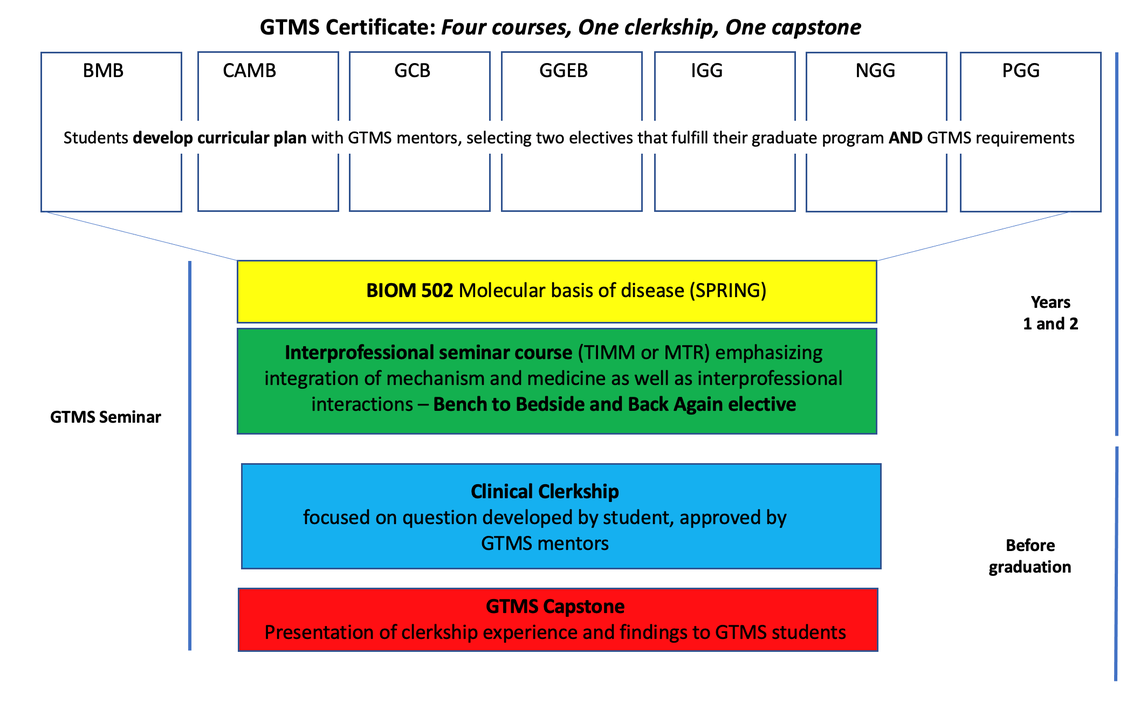
All GTMS Students
- Attend monthly GTMS Seminars: Informal monthly mentoring meetings to hear case studies, meet patients, develop medical vocabulary, communicate updates, and build community. In September of each year, second year GTMS students generate a schedule, identifying topics of interest and speakers they would like to hear discuss case studies and basic/clinical science collaborations.
Note that GTMS students who have not completed their capstone project must attend at least 80% of the monthly GTMS seminars. Those who have completed their capstone are very welcome to attend the seminar. Once a year GTMS mentors will meet individually with students to discuss progress and plans.
Year 1
- Develop curricular plan with GTMS mentors
- Overview of requirements in first semester
- Individual meetings with Jonathan and Jenni in the second semester
- Identify two electives that fulfill your GTMS curricular plan goals AND fulfill graduate group requirements
Year 2
- Finalize curricular plan
- Individual meetings with mentors in fall of first semester – submit description of courses that have/will fulfill GTMS requirements with short description of their relevance to your goals on Canvas.
- Finish course work, including
- BIOM 5020: Molecular Basis of Disease (Spring)
- One “Bench to Bedside” elective (examples include, but are not limited to, TIMM or ITMAT courses below)
- Topics in Molecular Medicine (CAMB 5420-402) – Fall
- Introduction to Clinical and Translational Research (MTR/REG 5100) – Fall
- Disease Measurement (MTR 6030) - Fall
- New Trends in Medicine and Vaccine Discovery (REG 6220) - Fall
- Manufacturing Novel Therapies & Imaging Agents (REG 6250) - Fall
- Introduction to Drug Development (REG 6120) – Fall and Spring
- Scientific and Ethical Conduct (MTR 6040) – Spring
- Post-Approval Maintenance of Drugs, Biologics, and Devices (REG 6150) – Spring
- Introduction to Vaccine Development (REG 6180) - Spring
- Medical Entrepreneurship: Commercializing Translational Science (MTR 6200) – Spring
- Cell and Gene Therapy (MTR 6210) – Spring
- Contribute to development of GTMS Seminar schedule
Year 3+
- Meet with Jonathan and Jenni as a group in the summer after prelims to discuss progress and clerkship opportunities
Before graduation – in a time determined by student and mentors
- Thesis Committee: Each student in the GTMS is strongly encouraged to include a clinician (MD or MD-PhD) as one of the members of the thesis committee.
- Clinical Clerkship: GTMS students are required to do a 40-hour clinical or translational (biotech or pharma) clerkship related to their area of research interest. They will meet with GTMS mentors to discuss their research focus and submit, for approval, a proposal that identifies a question that they will pursue during the clerkship. The clerkship may be completed as fits the student's schedule and the nature of the clerkship (eight hours a day for five days, four hours a week for 10 weeks, etc.). We suggest that students wait until they are settled in their thesis lab to identify clerkship activities.
- GTMS Capstone: At the end of your clerkship, you will submit a journal describing their findings in a format relevant to the clerkship’s focus. You will also present your clerkship experiences and findings to other students in the program as part of the GTMS Seminar series.

