Home

Professor of Neurology,
Chair, Neurology Department
Mission Statement
The Jensen lab utilizes multiple research platforms to explore the key role that synaptic changes play in neurological disease. We have previously shown that alterations in the excitatory: inhibitory balance in critical neuronal circuits contributes to neurodevelopmental disorders, epilepsy, and neurodegeneration. We utilize a combination of in vitro and in vivo preclinical models, as well as interrogation of human tissue, to examine cellular, protein and genetic changes that potentially represent novel therapeutic targets for translations.
A longstanding interest of the lab has been the pathophysiology of early life seizures and their effects on later life cognition and epilepsy risk. Neonatal seizures are refractory to medications that are effective in adulthood, and we used preclinical models and human tissue to show that targets of these medications were differentially, and often minimally, expressed in the neonatal brain. A series of investigations have identified that seizures at an early age produce long lasting changes in glutamatergic and GABAergic synapses, along with alteration modification of chloride transporters regulating the excitatory/inhibitory capacity of GABA receptors. Our work contributed to a clinical trial of the NKCC1 Cl/K co-transporter inhibitor in neonatal seizures (clinical trials.gov/NCT00830531), one of the first clinical trials to test the effects of modulating an “age-specific” therapeutic target.
Another main focus of our lab is to study the effects of seizures on learning, memory and social behavior. Utilizing both neurodevelopmental as well as later life epilepsy models, we have revealed that seizures modulate excitatory glutamate receptors to occlude synaptic plasticity, known to underlie learning and memory. We have shown that this is also the case in the developing brain, where seizures can impair critical period plasticity. Subsequently, we found that seizure activity results in post-translational modification of the AMPA subtype of glutamate receptor, increasing the expression of GluA2-lacking calcium-permeable AMPA receptors on principal neurons in hippocampus and neocortex, and subcellular signaling including the mTOR pathway. We also showed that post-seizure treatment with inhibitors of these receptors or mTOR inhibitors rescue this phenomenon in both epilepsy as well as in certain neurodevelopmental disorder models of autism associated with epilepsy.
Given the recent clinical data showing an association of epilepsy with neurodegenerative disorders, we have recently explored alterations in synaptic function in expression that can contribute to hyperexcitable circuits in preclinical models of dementia and human brain tissue from patients with dementia. We have shown that hyperexcitability can activate subcellular signaling pathways that associate mTOR with amyloid and tau accumulation. Our preclinical studies have revealed that mTOR inhibitors can prevent seizure induced exacerbation of neuropathology and cognition in preclinical models of dementia.
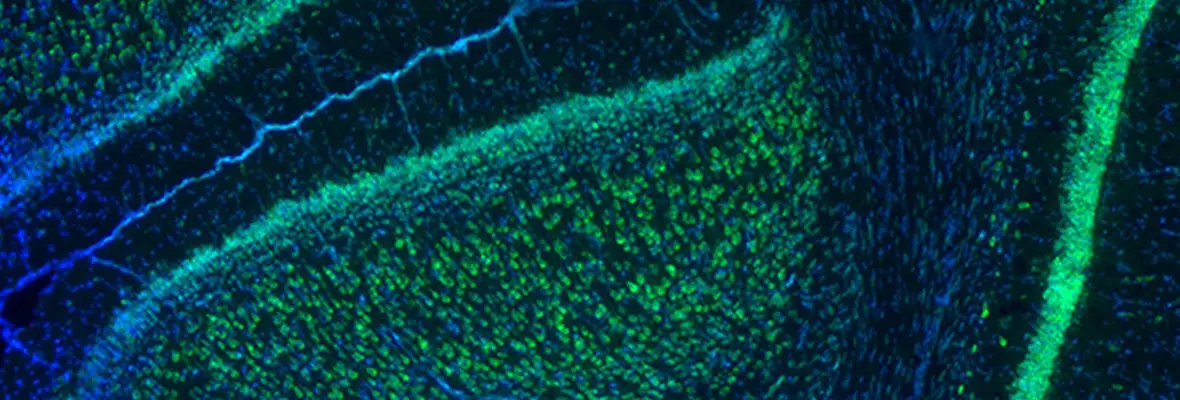
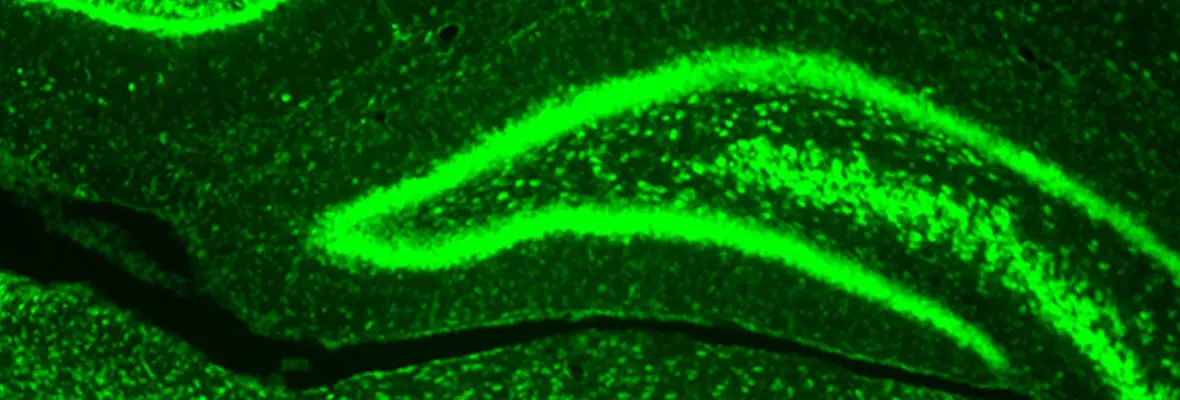
Our research methodologies include both in vivo and in vitro rodent models (mouse and rat), in vitro electrophysiology, optogenetic stimulation, intact animal EEG, MRI, and neurobehavioral studies. Cellular and molecular studies include immunohistochemistry, ISH, clarity, confocal and light sheet fluorescent microscopy, western blot and proteomics, RNA expression, FACS sorting, RNAscope as applied to both brain tissue from preclinical model, and human tissue samples.
Latest News
-
Rhythm of "Detox" and Feeding Genes in Fruitflies and Mice Coordinated by Neuropeptide, According to Penn Study
Tuesday, May 17, 2016
Findings have implications for the timing of medication regimens and circadian rhythm disorders