About Pancreatic Cancer
Table of Contents
The Clinical Challenge
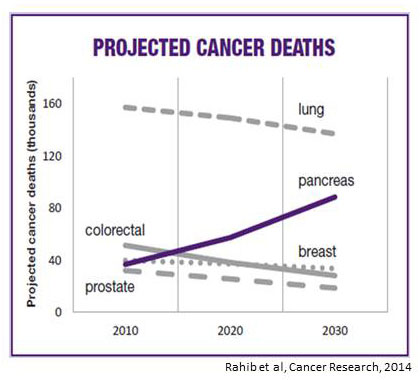
Pancreatic cancer is currently the third leading cause of cancer death in the United States, and epidemiologic projections indicate that it will be second only to lung cancer in its lethality by 2025. Two factors contribute to this high mortality: 1) the fact that most cancers have already spread (metastasized) at the time of diagnosis; and 2) a lack of effective therapies for metastatic pancreatic cancer. Thus, the major challenges for research are to develop better methods for early detection and novel therapies for treatment of advanced cases.
Risk Factors
The average lifetime risk of developing pancreatic cancer is approximately 1 in 67 (1.5%). Pancreatic cancer is more common with increasing age, although rarely, the disease strikes individuals in their 30s and 40s. There is no single cause of pancreatic cancer, but several environmental factors are associated with an increased risk of developing pancreatic cancer. These include tobacco use, obesity, African American race, chronic pancreatitis, and a strong family history (see Genetics). In addition, there is an association between pancreatic cancer and type 2 (adult onset) diabetes. Although the basis for this association is not known, several thought leaders believe that the diabetes in these cases is caused by the cancer (although the diabetes diagnosis may precede the cancer diagnosis).
Cellular Changes
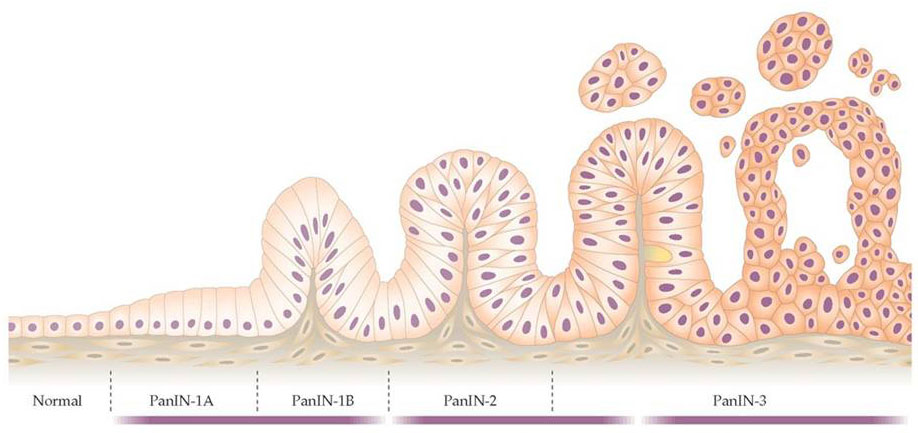
Like most cancers, pancreatic cancer arises from a series of genetic changes (mutations) leading to abnormal cell growth. A number of tissue changes have been recognized to serve as precursor lesions to pancreatic cancer, including PanINs (pancreatic intraepithelial neoplasias), IPMNs (intraductal papillary mucinous neoplasmas), and MCNs (mucinous cystic neoplasmas). These changes often occur at the microscopic level, and thus are not easily recognizable radiographically, although IPMNs and MCNs can sometimes be diagnosed by CT scan orendoscopy. These cellular changes are brought about by changes in genes (either gene mutations or epigenetic changes leading to altered gene expression) and compounded by changes in the stroma (fibroblasts, blood vessels, and white blood cells). With time, these events lead to more and more abnormal cellular behavior, leading to uncontrolled growth and invasive spread.
Genetics
Cancer is a genetic disease, meaning that it is caused by alterations in gene function. Cancer genes fall into two main categories: oncogenes, which drive cell growth and become activated by mutation, and tumor suppressor genes which inhibit cell growth and become inactivated by mutation. The spectrum of mutations giving rise to cancer varies from tumor to tumor, but several genes are reproducibly mutated in a large fraction of pancreatic cancers (Table 1). The Kras oncogene and the p53 tumor suppressor gene are mutated in the majority of pancreatic cancers. Other frequently mutated genes include Cdkn2a, SMAD4, and and TGFBR2. With the exception of Kras, all of these frequently mutated genes are tumor suppressor genes that have already been lost in the cancer cells, and thus they do not represent viable targets for therapy. The Kras oncogene has been the focus of great attention by the National Cancer Institute as a cancer target, but to date, there has been little progress in inhibiting this oncogene.
Familial Pancreatic Cancer
The majority of individuals with pancreatic cancer do not have a family history. However, approximately 5-10% of patients do have a family history, and many of these cases are associated with inherited mutations. Individuals with inherited mutations in BRCA2 or Palb2 — genes involved in the repair of damaged DNA — are at increased rick of pancreatic cancer, as are those with mutations in Stk1 (Peutz-Jehger“s syndrome), APC (familial adenomatous polyposis), and PRSS1 (hereditary pancreatitis). Individuals in families with any of these inherited syndromes, or with a high incidence of pancreatic cancer (more than 2-3 family members or a family member diagnosed before the age of 40), may have a genetic predisposition and could benefit from genetic screening. In general, individuals with a single family member diagnosed with pancreatic cancer are not at increased risk compared to the general population.

