Amaravadi Lab
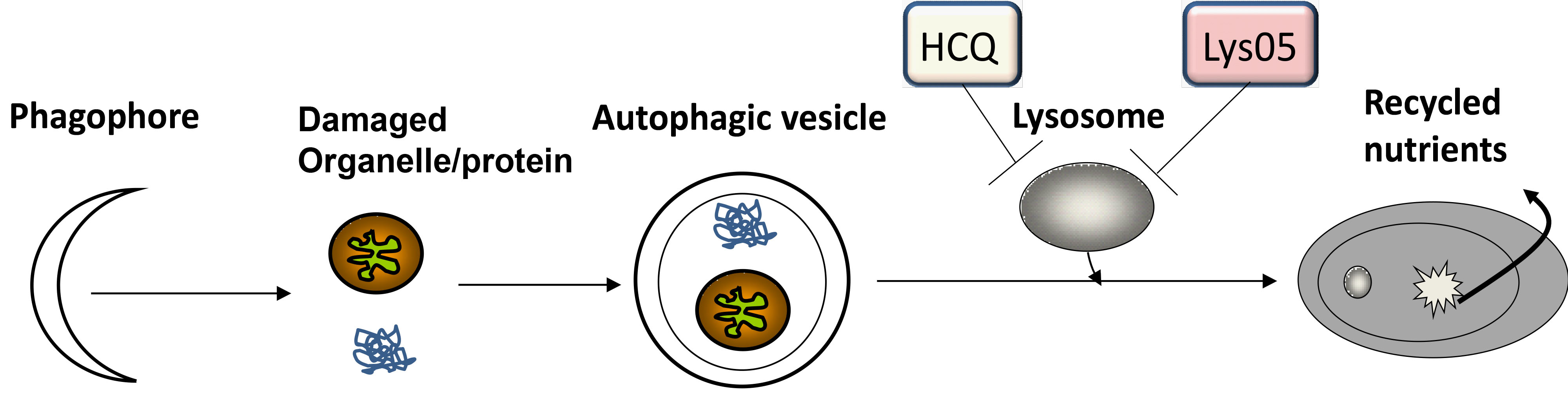
The Amaravadi Lab focuses on the role of autophagy in cancer therapy. Cancer therapy has made major strides in the past 20 years, but eventually most cancers have the ability to survive the stress of cancer therapy and recur, leading to suffering and death. We and others have demonstrated that autophagy, the process by which organelles such as mitochondria and proteins are internally digested and recycled, promotes cancer cell survival in advanced cancers, and is a potentially key resistance mechanism that cancers use to survive cancer therapy. Our lab collaborates closely with other basic scientists, clinical researchers, biotechnology and pharmaceutical companies so that we can test our hypotheses in cell lines, 3D culture, mouse models, and patients. We conduct basic laboratory research to understand fundamental aspects of autophagy in cancer biology, but we also have a translational focus. We are actively conducting innovative clinical trials intended to modulate autophagy to enhance effectiveness of existing therapies. Our funding comes largely from the National Cancer Institute, focused pharmaceutical collaborations, and private donations from patients and fundraising programs.
Current Projects
Can more potent and specific lysosomal autophagy inhibitors be identified?
Working with the laboratory of Dr. Jeffrey Winkler (Merriam Professor of Organic Chemistry Department of Chemistry, School of the Arts and Sciences University of Pennsylvania) we have designed, synthesized and tested the first series of dimeric chloroquine derivatives. We call these compounds Lys01 derivatives (see Mcafee et al PNAS 2012). We have identified dozens of potent derivatives (Rebecca et al. Cancer Discovery 2017; Rebecca et al. Cancer Discovery 2019) and identified the molecular target of chloroquine derivatives as palmitoyl-protein thioesterase 1 (PPT1). PPT1 inhibitors are being developed for clinical trials by Pinpoint Therapeutics, Inc.
What are the effects of lysosomal autophagy inhibition in specific cell types within the tumor microenvironment?
We recently showed that PPT1 inhibition in tumor associated macrophages enables T cell mediated killing of melanoma tumors ( Sharma et al. JCI Insight 2020). Numerous groups have demonstrated that autophagy inhibition at other nodes in the pathway augments anti tumor immunity. More work is needed to understand how best to use autophagy inhibitors in the context of immunotherapy.
What is the role of PPT1 in tumorigenesis?
Using CRISPR technology we have created novel tools to study the role of PPT1 in tumorigenesis
Can we find the cancers and patients most likely to benefit from lysosomal autophagy inhibition?
Using -omics platforms we are investigating, genetic and epigenetic determinants of sensitivity to chloroquine derivatives in a broad panel of tumor cell lines and patient tissues. This work is in collaboration with investigators from the the Abramson Family Cancer Research Institute and the Center for Clinical Epidemiology and Biostatistics. Preliminary findings were published (Piao Autophagy 2017), but we continue to investigate candidate markers of sensitivity in human tumor samples.
Can proteins that are secreted in an autophagy dependent manner be used to develop biomarkers of response to autophagy inhibitors?
Working with laboratory of Dr. David Speicher (Caspar Wistar Professor of Computational and Systems Biology, Wistar Institute), we have probed the secretome of melanoma cells grown in 3D culture and have identified autophagy dependent secreted proteins (Kraya et al Autophagy 2015). We are currently determining of effects of therapeutics that are known to modulate autophagy on secretome profiles. We are working on both small molecule targeted therapeutics and immunotherapies in immunocompetent mouse models.
How exactly does targeted therapy activate a cytoprotective autophagy response?
Working with the laboratory of Dr. Costas Koumenis (Professor and Vice Chair for Research Department of Radiation Oncology, University of Pennsylvania) we previously determined that BRAF inhibitors activate cytoprotective autophagy through an ER stress response( Ma et al JCI 2014). We leveraged our access to patient derived xenografts from patients that have progressed on BRAF inhibitors to determine the molecular underpinnings of the MAPK-ER stress-autophagy signaling. We have identified a new step in this pathway that involves druggable targets, KSR2, SEC61, PERK, ATF4 (Ojha et al. Cancer Discovery 2019) . We anticipate this work will uncover potential new combination therapies for BRAF mutant melanoma and other BRAF mutant cancers.
Announcement
- Amanda Versace accepted to the Cell and Molecular Biology graduate program at the University of Pennsylvania
- Dr. Amaravadi was inducted into the Association of American Physicians in 2023
- Dr. Amaravadi becomes the Associate Director for Translational Research at the Abramson Cancer Center
- Lazlo Nziga joined the lab as a PASS student for the summer of 2021/2022 and was accepted into UPENN medical school
- Jennifer Lee was in the lab from 2018-2022 and was accepted to Johns Hopkins Medical School
- Dr. Amaravadi is the co-Director of the newly funded Wistar/UPENN SPORE in Skin Cancer. https://www.news-medical.net/news/20210922/Penn-Medicine-and-Wistar-secure-24117-million-SPORE-grant-for-three-melanoma-research-projects.aspx
- Cynthia Chude, former Lab member becomes starts an MDPhD program as the first Meharry-Wharton-Leonard Davis Institute scholar: https://ldi.upenn.edu/our-work/research-updates/new-wharton-school-meharry-college-joined-md-phd-program-welcomes-first-scholar/
Spotlight
Jennifer Lee was welcomed into the Amaravadi lab in May 2017. Jennifer graduated from the Shipley School and spent her summer before college in our lab. She joined the University of Pennsylvania in the fall of 2017. Jennifer’s first research experience was during the summer of 2016.jpg) , when she interned at the Boston University Medical Center. There, she was exposed to the concept of autophagy in relation to diabetes. Afterwards, Lee earned research accolades from the Intel STS Competition. Jennifer has been working in the Amaravadi Lab each summer and has receieved a number of grants including the University Scholars Award to support this work. She has made major contributions to the lab and has become a co-author on a number of papers. Jennifer is excited to have the privilege to join the lab and contribute to the greater health of society. She finds the nexus between the macroscopic and microscopic events in the human body fascinating, and as a future woman in the STEM field, she hopes to inspire other young women to pursue a career in the science field.
, when she interned at the Boston University Medical Center. There, she was exposed to the concept of autophagy in relation to diabetes. Afterwards, Lee earned research accolades from the Intel STS Competition. Jennifer has been working in the Amaravadi Lab each summer and has receieved a number of grants including the University Scholars Award to support this work. She has made major contributions to the lab and has become a co-author on a number of papers. Jennifer is excited to have the privilege to join the lab and contribute to the greater health of society. She finds the nexus between the macroscopic and microscopic events in the human body fascinating, and as a future woman in the STEM field, she hopes to inspire other young women to pursue a career in the science field.