Archived Featured Faculty: Andrew Tsourkas
 Andrew Tsourkas, Ph.D.
Andrew Tsourkas, Ph.D.
Professor and Undergraduate Chair
Department of Bioengineering
University of Pennsylvania
Telephone: 215-898-8167
Email: atsourk@seas.upenn.edu
Dr. Andrew Tsourkas received his Ph.D. in Biomedical Engineering from Georgia Tech/Emory University and performed his post-doctoral training in cellular and molecular imaging at Mass. General Hospital, Harvard University. The overall goal of his research program is to develop targeted imaging agents for early cancer detection and targeted therapeutics for more personalized and efficient treatment of cancer. Over the past decade, his lab has developed magnetic resonance contrast agents that can detect tumors less than a millimeter in size, multifunctional nanoparticles that can significantly improve the outcome of patients receiving radiation therapy, and antibody-based therapeutics that can trigger a patient’s own immune system to destroy malignancies. Dr. Tsourkas is an inventor on over a dozen patents and has recently founded a company AlphaThera to bring the antibody-based therapeutics developed in his lab to market. Dr. Tsourkas has forged strong relationships throughout the medical school and routinely collaborates with Radiologists, Hematologist, Immunologists, Pathologists, and Radiation-Oncologists. He is also the Undergraduate Chair of Bioengineering and teaches both undergraduate- and graduate-level courses in medical imaging. Several areas of research being pursued in the Tsourkas lab are highlighted below:
Highly Paramagnetic gadolinium (Gd)-based Nanoparticles
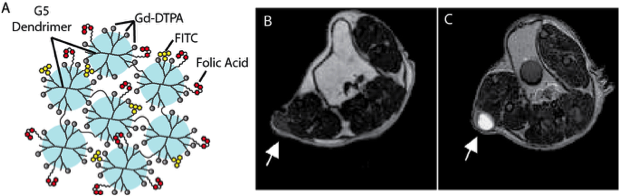
A major hurdle in developing receptor-targeted MR contrast agents is overcoming the relatively low sensitivity. To achieve the requisite high local concentrations of Gd within tissues that have a far lower concentration of biomarker targets, the Tsourkas lab developed Gd-labeled dendrimer nanoclusters (DNCs), a nanoplatform (~150 nm) designed to carry an extremely high Gd payload – 300,000 Gd atoms/particle (Fig. 1A). This led to an unprecedented relaxivity (r1) of 3.6 x 106 mM−1 sec−1 per nanoparticle at 1.4T. Because of their small size and the high density of Gd labeling, these agents can produce high signal to contrast within tumors in a molecularly-specific manner (Fig. 1B,C). More recently, the Tsourkas lab designed DNCs to include degradable linkages between the individual dendrimers, enabling more efficient excretion. It is hypothesized that this will reduce the likelihood of Nephrogenic Systemic Fibrosis.
- Cheng, Z., Thorek, D.L.J, Tsourkas, A. (2009) Porous polymersomes with encapsulated Gd-labeled dendrimers as highly efficient MRI contrast agents. Advanced Functional Materials, 19(23), 3753-3759.
- Cheng, Z., Thorek, D.L.J., Tsourkas, A. (2010) Gd-conjugated dendrimer nanoclusters as a tumor targeted T1 magnetic resonance imaging contrast agent. Angewandte Chemie International Edition, 49(2), 346-350. PMCID: PMC2862691
- Huang, C-H., Nwe, K., Al Zaki, A., Brechbiel, M. Tsourkas, A. (2012) Biodegradable polydisulfide dendrimer nanoclusters as MRI contrast agents. ACS Nano, 6(11), 9416-9424. PMCID: PMC3508381.
- Cheng, Z, Al Zaki, A., Aspinwall, C., Tsourkas, A. (2014) Stabilized porous liposomes with encapsulated Gd-labeled dextran as highly efficient MRI contrast agents. Chemical Communications, 50(19), 2502-2504. PMCID:PMC3947407.
Theranostic Agents for Radiation Therapy and Imaging
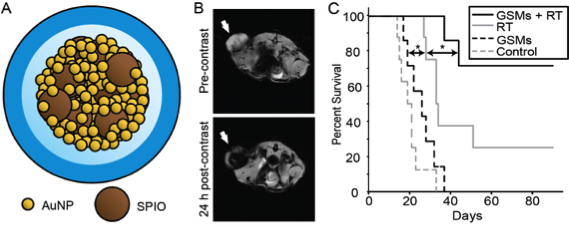
The Tsourkas lab recently developed a theranostic nanoplatform, consisting of gold- and superparamagnetic iron oxide (SPIO) nanoparticles, with well-aligned radiotherapeutic and diagnostic properties (Fig. 2A). When GSMs were intravenously administered into tumor-bearing mice selective tumoral accumulation enabled MR imaging of tumor margins (Fig. 2B). Subsequent irradiation led to improved survival, due to gold-mediated radiosensitization, compared to mice receiving radiation alone (Fig. 2C). Contrast-enhanced MR was predictive or tumor response, providing a promising mechanism to guide follow-up treatment.
- Joh, D.Y., Sun, L., Stangl, M., Al Zaki, A., Murty, S., Davis, J.J., Racharla, L., Bhang, D.H., Baumann, B.C., Alonso-Basanta, M., Ryeom, S.W., Kao, G.D., Tsourkas, A., Dorsey, J.F. (2013) Selective targeting of brain tumors with nanoparticle-induced radiosensitization. PLoS One, 8(4), e62425. PMCID: PMC3640092.
- Al Zaki, A., Joh, D., Cheng, Z., de Barros, A.L., Kao, G.D., Dorsey, J.F., Tsourkas, A. (2014) Gold-loaded polymeric micelles for computed tomography–guided radiation therapy treatment and radiosensitization. ACS Nano, 8(1), 104-112. PMCID: PMC3906892.
- McQuade*, C., Al Zaki*, A., Desai, Y., Vido, M., Sakhuja, T., Cheng, Z., Hickey, R., Joh, D., Park, S-J, Kao, G.D., Dorsey, J.F., Tsourkas, A. (2015). A multi-functional nanoplatform for imaging, radiotherapy, and the prediction of therapeutic response. Small, 11(7), 834-843 (*contributed equally). PMCID: PMC4329028.
- Sun L., Joh D., Stangl M., Al Zaki A., Murty S., Alonso-Basanta M., Kao G, Tsourkas A., Dorsey J.F. (2016) Theranostic application of mixed gold and iron oxide nanoparticle micelles in glioblastoma multiforme. Journal of Biomedical Nanotechnology, 12, 347-356.
Site-Specific Bioconjugate Chemistry
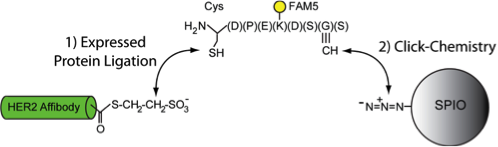
While bioconjugation techniques have steadily improved in recent years, the field is still plagued with inefficient conjugation reactions and/or the lack of site- specific coupling. To overcome this challenge, the Tsourkas lab recently created a suite of bioconjugation techniques that allow for the site-specific and efficient (~100%) functionalization of biomolecules. The approaches are simple, rapid, scalable and can be used with any targeting ligands ranging in size from a peptide to a full-length antibody. These bioconjugation strategies are being used to facilitate the attachment of targeting ligands to nanoparticles (e.g. Fig. 3) and to accelerate the production of advanced antibody-based therapeutics, including antibody-drug conjugates and bispecific antibodies.
- Hui, J.Z., Tamsen, S., Song, Y., Tsourkas, A. (2015) LASIC: Light activated site-specific conjugation of native IgGs. Bioconjugate Chemistry, 26(8), 1456-1460.
- Warden-Rothman, R., Caturegli, I., Popik, V., Tsourkas, A. (2013) Sortase-Tag Expressed Protein Ligation (STEPL): combining protein purification and site-specific bioconjugation into a single step. Analytical Chemistry, 85(22), 11090-11097. PMCID: PMC3843242.
- Hui, J.Z., Al Zaki, A., Cheng, Z., McNitt, C.D., Popik, V., Zhang, H., Luning Prak, E.T., Tsourkas, A. (2014) Facile method for the site-specific, covalent attachment of full-length IgG onto nanoparticles. Small, 10(16), 3354-3363. PMCID:PMC4142076.
- Hui, J.Z., Tsourkas, A. (2014) Optimization of photo-active Protein Z for fast and efficient site-specific conjugation of native IgG. Bioconjugate Chemistry, 25(9), 1709-1719. PMCID:PMC4166039.
Ratiometric BiMolecular Beacons:
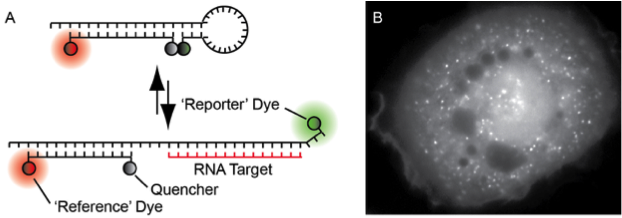
The Tsourkas lab recently developed a new probe for imaging RNA in living cells, ratiometric bimolecular beacon (RBMB; Fig. 4A), which exhibits a high signal-to-background and allows for RNA imaging for up to 24 hours. RBMBs can be efficiently delivered into a wide range of cells types via microporation, with little to no effect on cell viability, and quantitative measurements of gene expression can be acquired within 30 minutes. Moreover, the methodology is fairly insensitive to RBMB concentration and target RNA levels, since fluorescence from unbound RBMBs is efficiently quenched. When RBMBs are administered to cells at appropriate working concentrations, there is no effect on target gene or protein expression. It is envisioned that RBMBs will provide unique insight into RNA biology and allow for the study of a wide range of RNA behaviors.
- Zhang, X., Zajac, A.L., Huang, L., Behlke, M.A., Tsourkas, A. (2014) Imaging the directed transport of single engineered RNA transcripts in real-time using ratiometric bimolecular beacons. PLoS One. 9(1), e85813. PMCID:PMC3893274.
- Zhang, X., Song, Y., Shah, A.Y., Lekova, V., Raj, A., Huang, L., Behlke, M.A., Tsourkas, A. (2013) Quantitative Assessment of Ratiometric BiMolecular Beacons as a Tool for Imaging Single Engineered RNA Transcripts and Measuring Gene Expression in Living Cells. Nucleic Acids Research, 41(15), e152. PMCID: PMC3753654.
- Chen, A., Davydenko, O., Behlke, M.A., Tsourkas, A. (2010) Ratiometric BiMolecular Beacons for the sensitive detection of RNA in single living cells. Nucleic Acids Research, 38(14), e148. PMCID: PMC2919734
- Chen, A.K., Behlke, M.A., Tsourkas, A. (2008) Efficient cytosolic delivery of molecular beacon conjugates and flow cytometric analysis of target RNA. Nucleic Acids Research, 36(12), e69. PMCID: PMC2475621


