Archived Featured Faculty: Jason Burdick
Jason A. Burdick, Ph.D.
Robert D. Bent Professor of Bioengineering
Department of Bioengineering
University of Pennsylvania
Telephone: 215-898-8537
Email: burdick2@seas.upenn.edu
Dr. Jason A. Burdick received his PhD. in Chemical Engineering from the University of Colorado and completed post-doctoral training at the Massachusetts Institute of Technology prior to his arrival at Penn in 2005. Dr. Burdick’s research involves the development of hydrogels through techniques such as photocrosslinking and self-assembly and their processing using approaches such as electrospinning and 3D printing. The applications of his research range from controlling stem cell differentiation through material cues to fabricating scaffolding for regenerative medicine and tissue repair.
Dr. Burdick has been awarded a K22 Scholar Development and Career Transition Award through the National Institutes of Health, an Early Career Award through the Coulter Foundation, a National Science Foundation CAREER award, a Packard Fellowship in Science and Engineering, and an American Heart Association Established Investigator Award. He was recently awarded the Clemson Award through the Society for Biomaterials and the George H. Heilmeier Faculty Award for Excellence in Research. He is on the editorial boards of Tissue Engineering, Biofabrication, and Journal of Biomedical Materials Research A, and is an Associate Editor for ACS Biomaterials Science & Engineering.
Several areas of research being pursued by the Burdick laboratory are highlighted below:
Therapeutic Hydrogel Delivery for treating Myocardial Infarction
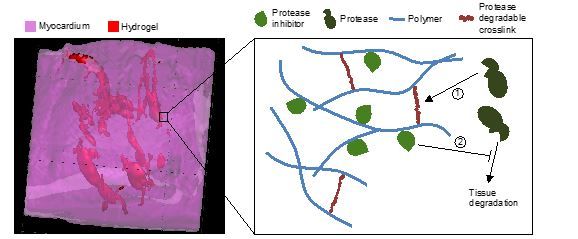
There are numerous biological targets to treat cardiac tissue after myocardial infarction (MI) to limit the adverse left ventricular remodeling processes that occur. Hydrogels permit the alteration of local tissue mechanics, as well as the delivery of cells and therapeutic molecules. For example, matrix metalloproteinase (MMP) levels increase drastically after MI and their corresponding tissue inhibitors of MMPs (TIMPs) decrease. We designed injectable hydrogels that localize placement of depots of one inhibitor (i.e., TIMP-3) within cardiac tissue after MI. Moreover, the hydrogels were designed so that they sequester the TIMP-3 through electrostatics and only degrade in the presence of MMPs. These “smart” features permitted the on-demand degradation and TIMP-3 release from the hydrogels, to further address the spatiotemporal heterogeneity in enzyme levels within tissues and between patients. Beyond protease inhibitor delivery, we have used biodegradable hydrogels to deliver a variety of factors, such as chemokines that enhance the homing of stem cells to the injury site or molecules (e.g., miRNA) that initiate cardiomyocyte proliferation towards a regenerative approach.
1. B.P. Purcell, D. Lobb, M.B. Charati, S.M. Dorsey, R.J. Wade, K.N. Zellars, H. Doviak, S. Pettaway, C.B. Logdon, J.A. Shuman, C. Novak, J.H. Gorman, R.C. Gorman, F.G. Spinale, J.A. Burdick, Injectable and Bioresponsive Hydrogels for On-Demand Matrix Metalloproteinase Inhibition, Nature Materials, 13:653-661, 2014. PMCID: PMC4031269
2. V. Ramjee, D. Li, L.J. Manderfield, F. Liu, K.A. Engleka, H. Aghajanian, C.B. Rodell, W. Lu, V. Ho, T. Wang, L. Li, A. Singh, D. Cibi, J.A. Burdick, M.K. Singh, R. Jain, J.A. Epstein, Epicardial YAP/TAZ Orchestrate an Immune Suppressive Response following MI, Journal of Clinical Investigation, 127:899-911, 2017. PMCID: PMC5330722
3. L.L. Wang, Y. Liu, J.J. Chung, T. Wang, A.C. Gaffey, M. Lu, C.A. Cavanaugh, S. Zhou, R. Kanade, P. Atluri, E.E. Morrisey, J.A. Burdick, Sustained miRNA Delivery from an Injectable Hydrogel Promotes Cardiomyocyte Proliferation and Functional Regeneration after Ischaemic Injury, Nature Biomedical Engineering, 1: 983-992, 2017. PMCID: PMC5773070
Controlling Cell Differentiation in 3D Hydrogels
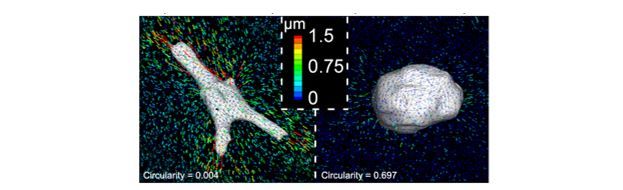
Hydrogels are useful in both direct applications in regenerative medicine as cell carriers and for the investigation of fundamental questions related to the cellular microenvironment. For example, the majority of studies on mechanotransduction have involved seeding of cells atop films of hydrogels, where mechanics can be tuned through changes in material crosslink density. We investigated MSC fate and cell-generated tractions using 3-D traction force microscopy within covalently crosslinked hydrogels and showed that cells remained rounded and maintained low traction irrespective of the mechanical magnitude; however, when crosslinks that could be cleaved by cellular enzymes were introduced and cells could spread, they were able to pull on the material and undergo differentiation down an osteogenic lineage. These findings are very significant in that they highlight how simple changes in the hydrogel system used can lead to large differences in how the environment is perceived by cells and we have followed this up by investigating how signaling (e.g., YAP/TAZ) is influenced by these signals. In addition to hydrogel degradation, other features such as the structure of the material can play a role in cell behavior. Towards this, we have investigated how fibrillar hydrogel microenvironments influence cell behavior.
1. S. Khetan, M. Guvendiren, W.R. Legant, D.M. Cohen, C.S. Chen, J.A. Burdick, Degradation-mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-dimensional Hydrogels, Nature Materials, 12:458-465, 2013. PMCID: PMC3633615
2. B.M. Baker, B. Trappmann, W.Y. Wang, M.S. Sakar, I.L. Kim, V.B. Shenoy, J.A. Burdick, C.S. Chen, Cell-Mediated Fiber Recruitment Drives Extracellular Matrix Mechanosensing in Engineered Fibrillar Microenvironments, Nature Materials, 14:1262-1268, 2015. PMCID: PMC4654682S.R.
3. Caliari, S.L. Vega, M. Kwon, E.M. Soulas, J.A. Burdick, Dimensionality and Spreading Influence MSC YAP/TAZ Signaling in Hydrogel Environments, Biomaterials, 103:314-323, 2016. PMCID: PMC4963302
Shear-thinning and Self-Healing Hydrogels
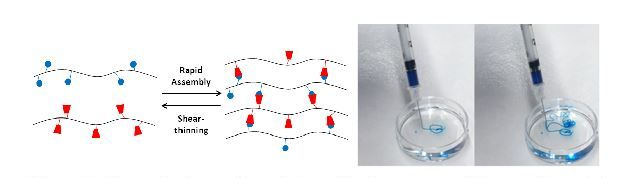
Self-assembling and shear-thinning are useful as injectable materials for biomedical applications and for 3D-printing. These properties are due to the nature of the bonds used in the supramolecular assembly, which can be disrupted through mechanical forces and then re-form due to their high affinity. In one example, guest-host molecules (e.g., cyclodextrin as host, adamantane as guest) that meet these bond criteria are coupled to polysaccharides to form assemblies with tunable properties (e.g., mechanics, degradation, molecule release kinetics). The unique self-assembling and shear-thinning properties of this hydrogel allow it to be injectable through either a syringe or even through a catheter to precisely position the material in a wide range of tissues (e.g., liver or heart). Thus, the control over the material degradation and the ability to localize in tissue provides unique opportunities to control the spatiotemporal presentation of biological molecules in vivo. In addition to use as injectable therapeutics, the shear-thinning and self-healing properties allow the materials to be 3D printed through extrusion-based techniques, towards the generation of complex scaffolds with precise positioning of materials and cells.
1. C.B. Rodell, J.W. MacArthur, S.M. Dorsey, R.J. Wade, L.L. Wang, Y.J. Woo, J.A. Burdick, Shear-thinning Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo, Advanced Functional Materials, 25:636-644, 2015. PMCID: PMC4624407
2. C.B. Rodell, M.E. Lee, H. Wang, S. Takebayashi, T. Takayama, T. Kawaura, J.S. Arkles, N.N. Dusaj, S.M. Dorsey, W.R.T. Witschey, J.J. Pilla, J.H. Gorman, J.F. Wenk, J.A. Burdick, R.C. Gorman, Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit LV Remodeling, Circulation: Cardiovascular Interventions, 9:e004058, 2016. PMCID: PMC5123705
3. C.B. Highley, C.B. Rodell, J.A. Burdick, Direct 3D Printing of Shear-thinning Hydrogels into Self-healing Hydrogels, Advanced Materials, 27:5075-5079, 2015.
4. L. Ouyang, C.B. Highley, W. Sun, J.A. Burdick, A Generalizable Strategy for the 3D Printing of Hydrogels from Non-viscous Photocrosslinkable Inks, Advanced Materials, 29:1604983, 2017.


