Media
Veteran Prostate Cancer: New Genetic Clues Found
Precision Oncology: Genomic Profiling Reveals Key Differences in Prostate Cancer Among Veterans
A large-scale study highlights the importance of genomic testing in achieving equitable cancer care for all populations.
Groundbreaking Research Unveils Genetic Disparities, Equal Outcomes
A recent study, the largest of its kind, has shed light on the genomic differences in metastatic prostate cancer between non-Hispanic Black and non-Hispanic white veterans. The research,conducted by experts from Moffitt Cancer Center,the University of Pennsylvania,the University of California Los Angeles,and the Veterans Affairs (VA) National Precision Oncology Program,analyzed data from over 5,000 U.S. veterans.The findings, published in JAMA Network Open, reveal that while there are distinct biological variations, survival outcomes are similar when both groups have equal access to care.
Did You Know?
Prostate cancer is the second leading cause of cancer death in American men, with African American men facing a disproportionately higher risk.
The study, which spanned from 2019 to 2023, utilized next-generation sequencing to analyze tumor samples. researchers discovered that non-Hispanic Black veterans exhibited higher rates of actionable immunotherapy targets, while non-Hispanic white veterans showed more frequent alterations in androgen receptor signaling and DNA repair pathways.
The Promise of Precision Oncology
The study underscores the potential of precision oncology in addressing cancer disparities. These results affirm that precision oncology can be a powerful tool for achieving equitable cancer care, said Dr. Kosj Yamoah, M.D., Ph.D., senior author and chair of the Radiation Oncology Program at Moffitt. By using genomic testing to guide therapy selection, we can match patients to treatments based on their tumor biology, not race.
Pro Tip
Genomic testing can help identify specific mutations in cancer cells, allowing doctors to tailor treatment plans for individual patients.
Dr. Isla Garraway, M.D., Ph.D., co-senior author and director of research in the Urology Department at UCLA Health, emphasized the importance of challenging historical disparities.
This research reinforces that we must not let historical disparities define modern care.Instead, by prioritizing access to genomic tools, we can drive meaningful change in how prostate cancer is treated across all populations.
dr. Isla Garraway, M.D., Ph.D., co-senior author and director of research in the Urology Department at UCLA Health
key Findings at a glance
- Non-Hispanic Black veterans were significantly more likely to have genomic alterations associated with immunotherapy benefit, such as microsatellite instability.
- Non-Hispanic white veterans had higher rates of mutations in DNA repair genes and the androgen receptor axis,which may influence responsiveness to hormonal therapies.
- Tumor suppressor gene alterations were linked to worse survival in both groups.
- No biomarker was found that shoudl be excluded from testing based on race.
Inclusion and Future Directions
The study’s cohort, comprising 36% non-Hispanic Black veterans, marks a meaningful betterment in inclusivity compared to previous genomic studies. Researchers stress the need to continue expanding access to next-generation sequencing testing and ensuring that underrepresented groups are included in precision oncology research and clinical trials.
This study shows that when we remove barriers to care and apply precision medicine equitably, we can improve outcomes for all patients, said Dr.Kara Maxwell, M.D., Ph.D., co-senior author and assistant professor of medicine at the University of Pennsylvania’s Perelman School of Medicine.
Frequently Asked Questions
What is genomic profiling?
Genomic profiling involves analyzing the DNA of cancer cells to identify specific mutations that can guide treatment decisions.
Why is this study important?
this study highlights the importance of precision oncology in addressing cancer disparities and ensuring equitable care for all populations.
What are the implications of the study’s findings?
The findings suggest that by using genomic testing to guide therapy selection, doctors can match patients to treatments based on their tumor biology, not race.
More facts: Luca F. Valle et al, Somatic Tumor Next-Generation Sequencing in US Veterans With Metastatic Prostate Cancer, JAMA Network Open (2025). DOI: 10.1001/JamanetWorkOPen.2025.9119
Close
Groundbreaking Genomic Study of Veterans with Metastatic Prostate Cancer Uncovers Vital Advances for Precision Medicine
In a groundbreaking genomic study spearheaded by leading cancer research institutions, including Moffitt Cancer Center, the University of Pennsylvania, and UCLA Health, a comprehensive analysis was conducted on metastatic prostate cancer among U.S. veterans, focusing specifically on non-Hispanic Black and non-Hispanic white populations. This extensive research effort, published recently in a major peer-reviewed journal, represents the largest clinical genomic profiling study of its kind in non-Hispanic Black men, and it challenges longstanding assumptions about biological differences and treatment disparities in prostate cancer.
Prostate cancer remains one of the most prevalent cancers affecting men worldwide, and its metastatic form particularly poses significant treatment challenges. Historically, clinical outcomes have varied across racial groups, often attributed to both biological and socio-economic factors. However, this new study leveraged next-generation sequencing (NGS) technology to unravel the complex genomic landscape of metastatic prostate tumors within an equal-access healthcare system—the Veterans Affairs (VA) healthcare network—thereby isolating biological variables from confounding factors related to differential access to care.
Between 2019 and 2023, the study collected data from over 5,000 veterans who underwent tumor profiling using advanced somatic mutation sequencing technologies. By focusing on an equal-access cohort, researchers were able to carefully examine molecular differences without the noise introduced by disparities in healthcare availability or socioeconomic status. This approach allowed the team to delineate the true tumor biology differences between non-Hispanic Black and white veterans with metastatic prostate cancer.
The analysis revealed that non-Hispanic Black veterans exhibited a higher prevalence of genomic alterations linked to potential responsiveness to immunotherapy, including markers of microsatellite instability (MSI). MSI is indicative of defective DNA mismatch repair mechanisms, which have emerged as predictive biomarkers for immune checkpoint inhibitor efficacy in several cancer types. This finding underscores the potential for immunotherapeutic strategies to be particularly beneficial in this patient subgroup.
Conversely, non-Hispanic white veterans were more likely to harbor alterations affecting the androgen receptor signaling axis and DNA repair pathways. Mutations in genes responsible for DNA repair, such as BRCA1/2 and others within the homologous recombination repair (HRR) pathway, may sensitize tumors to treatments like PARP inhibitors and hormonal therapies, which target androgen signaling. These molecular signatures hint at a biological divergence in tumor evolution and therapeutic vulnerabilities between these racial groups.
Despite these distinct genomic landscapes, the study importantly observed no significant difference in overall survival between the two cohorts within the VA system. This suggests that when diagnostic and therapeutic resources are equitably distributed and used to guide precision medicine approaches, racial disparities in clinical outcomes can be effectively mitigated. The findings highlight the transformative potential of precision oncology when supported by equal access to high-quality care and genomic-guided therapy.
A crucial insight from the study is the lack of any biomarker that should be excluded from genomic testing based on a patient’s race. This challenges a previously held notion in some clinical circles that race-based testing strategies might optimize resource allocation. Instead, comprehensive genomic profiling for all patients stands as the best practice, ensuring that actionable mutations are not overlooked and that treatment plans are individualized based on tumor biology rather than demographic factors.
The researchers also identified that alterations in tumor suppressor genes—central regulators of cell cycle and apoptosis—were associated with poorer survival outcomes irrespective of racial background. This finding underscores the universal prognostic significance of these genomic aberrations and points towards the need to develop targeted therapeutics aimed at these pathways to improve patient prognosis.
The study’s cohort was notably more diverse than previous genomic studies, with non-Hispanic Black veterans making up 36% of participants—an important step forward in addressing historical underrepresentation in cancer research. This inclusion enriches the robustness and applicability of the findings and emphasizes the critical need to incorporate diverse populations in future oncology research and clinical trials to ensure equitable advances in cancer treatment.
At the helm of this investigation, experts emphasized the broader implications of these results for the field of cancer care. The principal investigators argued convincingly that precision oncology, empowered by comprehensive genomic profiling and equitable healthcare access, holds the key to dismantling systemic disparities. This concept reframes the narrative around racial health disparities, placing the emphasis on removing access barriers and utilizing molecular insights to tailor therapies.
Moreover, the study exemplifies the power of next-generation sequencing technologies beyond academic curiosity; it is a vital clinical tool that can guide decisions about immunotherapy, hormonal therapy, and targeted agents tailored to the distinct mutational profiles of a patient’s tumor. By doing so, personalized treatment plans replace the outdated one-size-fits-all approach, promising better efficacy and reduced toxicity.
In conclusion, this landmark research clearly demonstrates that the integration of high-throughput genomic diagnostics with equitable healthcare provision can level the playing field for racially diverse populations suffering from metastatic prostate cancer. It sheds light on biological complexities while reinforcing health equity, ultimately calling for widespread adoption of comprehensive genomic testing and inclusive clinical trial designs.
As cancer research continues to evolve, these findings demand attention from clinicians, researchers, and policymakers alike, urging a commitment to precision oncology supported by accessible, equitable healthcare infrastructure. This study not only advances scientific knowledge but also offers hope to patients historically marginalized by healthcare systems, showing that equitable care combined with cutting-edge genomics can lead to equally improved outcomes across populations.
Subject of Research: People
Article Title: Somatic Tumor Next-Generation Sequencing in US Veterans With Metastatic Prostate Cancer
News Publication Date: 12-May-2025
Web References:
- JAMA Network Open article: http://dx.doi.org/10.1001/jamanetworkopen.2025.9119
- Moffitt Cancer Center: https://moffitt.org/
- UCLA Health: https://www.uclahealth.org/
References:
- Yamoah, K., Garraway, I., Maxwell, K., et al. (2025). Somatic Tumor Next-Generation Sequencing in US Veterans With Metastatic Prostate Cancer. JAMA Network Open. DOI: 10.1001/jamanetworkopen.2025.9119
Close
Cancer Risk in BRCA-Positive Men - Penn Medicine Physician Interviews
Cancer and surgery specialists Drs. Kara Maxwell, Daniel Lee, Jennifer Zhang, and Bryson Katona delve into the nuances of BRCA-positive breast, prostate, and pancreatic cancers in men. This wide-ranging discussion encompasses BRCA heritability, diagnostics, screening, risks, and treatment options.
Close
2025 Breakthroughs & Discoveries Panel
The Basser Center's 2025 Breakthroughs and Discoveries Panel brought together internationally renowned experts in the field of BRCA-related cancer research and care. Basser Center Executive Director Susan Domchek, MD, moderated the discussion which featured Patrick Sung, DPhil, of The University of Texas Health Science Center at San Antonio, Gareth Evans, MD, PhD, FRCP, FRCOG of Manchester Breast Centre, and Kara Maxwell, MD, PhD of Basser's Men & BRCA Program.
Close
New tool puts reproductive risk for BRCA carriers into perspective
New tool puts reproductive risk for BRCA carriers into perspective
Basser Center for BRCA researchers share a new resource for genetic counseling conversations related to cancer risk genes and family planning.
“I just wish someone had told me this was a possibility.”
Kara Maxwell, MD, PhD, distinctly remembers the moment she heard those words eight years ago from the mother of a child with Fanconi anemia (FA). Maxwell met her at a conference focused on the rare, recessive genetic bone marrow disorder that causes myriad medical challenges, including childhood cancer. While survival is improving, the average lifespan for FA is currently only 20 to 30 years.
FA is an inherited disorder that can happen when both parents carry mutations in certain genes associated with cancer risk, including BRCA1 or BRCA2. The woman that Maxwell met was a BRCA2 gene mutation carrier. She told Maxwell that she wouldn’t have changed anything about her pregnancy, but she just wished she’d been made aware of the fact that—in addition to personal cancer risks and the potential of passing down a gene mutation to their children—BRCA gene mutations can also create entirely new genetic issues, if two carriers have a child together.
“If one of my patients is already dealing with the difficult news of learning they’re a BRCA mutation carrier, I don’t ever want them to be surprised by having a child with an even more difficult diagnosis,” Maxwell said.
The risk is rare and nuanced. It takes a geneticist or a licensed genetic counselor to unravel and explain the probabilities. But, until recently, professionals did not have a comprehensive tool to reference for such discussions. In November, Maxwell, an assistant professor of Hematology-Oncology and Genetics, and her team published a resource for genetic professionals to help counsel individuals with cancer-related genetic mutations, like BRCA1/2, about reproductive risks.
A primer on cancer genetics
BRCA genes are among the most well-known genes linked to hereditary cancer risk. An individual with a mutation in the BRCA1 or BRCA2 gene has an increased lifetime risk of developing breast, ovarian, pancreatic, or advanced prostate cancer. These gene mutations are passed down through families. Men and women of all races and ethnicities can have a BRCA gene mutation, though they’re most common among certain groups, notably those of Ashkenazi Jewish ancestry.
But BRCA genes aren’t the only genes linked to an increase cancer risk. Cancer genetic testing typically involves a multi-gene panel test that also looks for mutations in other genes. For example, mutations in the genes MLH1, MSH2, MSH6, or PMS2, are linked to Lynch Syndrome, the most common cause of hereditary colorectal cancer.
“Everyone should take stock of their family cancer history and talk to their doctor to find out if genetic testing is recommended,” said Susan Domchek, MD, executive director of the Basser Center for BRCA at Penn Medicine. “If someone receives genetic testing and learns that they carry a cancer gene mutation, it may also have a major impact on their reproductive decision making.”
How cancer gene mutations affect reproductive planning
By definition, inherited gene mutations can be passed down to one’s children. A parent who has a mutation in a cancer risk gene has a 50 percent chance of passing it on to each of their children, and a 50 percent chance of passing on the normal copy of the gene. Men, as well as women, can inherit and pass on a mutation in a cancer risk gene to either a son or a daughter.
If both parents carry a mutation in a cancer risk gene, then not only does their child now have a 75 percent chance of inheriting the mutation, but the combination of the damaged genes can create new problems, in the form of life-shortening disorders that typically begin to cause symptoms in childhood. For example, in addition to childhood cancers, FA can cause developmental abnormalities, abnormal thumbs and/or facial features, and kidney dysfunction.
However, BRCA1/2 and other cancer risk genes haven’t traditionally been part of the carrier screening panels that are typically offered as part of standard reproductive planning.
“So, if you’re a genetic counselor talking to someone newly diagnosed as a carrier for a cancer risk gene mutation, how do you help them decide how much effort—time, money, resources, emotions— to spend on getting genetic testing for their partner? That’s the question we set out to answer,” Maxwell explained. “It’s not a simple decision, because testing for cancer risk genes is not free, and it requires effort and input.”
A tool to help inform reproductive counseling for cancer gene mutation carriers
Maxwell’s team created a resource that helps to put these risks into perspective. Their paper provides estimates across different genetic ancestries of the risk of being a carrier for a genetic variant that causes life-limiting severe pediatric syndromes, and the risk of your child actually having one of these conditions, if you’re also a carrier of the same mutation.
For example, if you’re a BRCA2 mutation carrier and your partner is of Latin American genetic ancestry, their risk of being a BRCA2 mutation carrier in the absence of family history is approximately 1:231 (0.4 percent) and so your risk of having a child with a pediatric disease caused by two BRCA2 gene mutations is about 1:923 (0.1 percent). Putting it into context, Maxwell notes, that’s a similar level of risk to having a child with Down syndrome at age 30 (about one in 1,000).
“This tool is valuable from the genetic counseling perspective. It provides clear, concise data tables you can use to quickly compute a more precise risk estimate for a potential pediatric condition occurring in offspring in the context of being a carrier of a single gene mutation," said first author Jacquelyn Powers, MS, LCGC, associate director of Genetic Counseling for the Cancer Service Line at Penn Medicine. “We hope this tool will increase and enhance reproductive risk discussions with adults who have hereditary cancer predisposition syndromes.”
Empowering patients with the data to make personal choice
What BRCA carriers and their partners decide to do with this information will look different for everyone. Some couples will decide it’s worth it to move forward with genetic testing for their partner. Others will feel relieved by low odds and decide it’s not necessary. Some people will feel reassured simply to have a better understanding of all the possibilities. Others may decide to explore options like preimplantation genetic testing to ensure their children do not inherit a cancer risk gene mutation.
“All of these reproductive decisions are layered with so many personal choices,” Maxwell said. “We want people to feel like they have all of the information they need to make those choices.”
Close
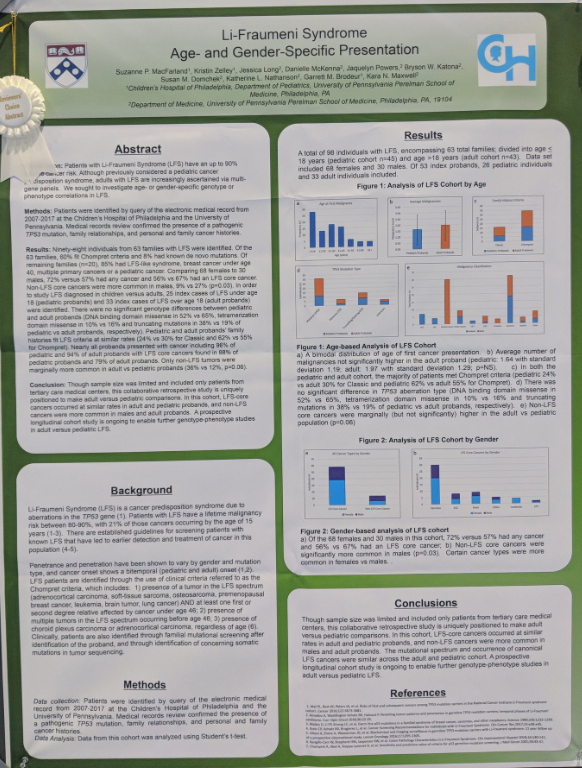
Confronting Li Fraumeni syndrome
Close
Dr Maxwell on a Study of Genetic Testing in Metastatic Prostate Cancer
Dr Maxwell on a Study of Genetic Testing in Metastatic Prostate Cancer
Kara N. Maxwell, MD, PhD, assistant professor of medicine (hematology-oncology), Department of Medicine, Perelman School of Medicine, University of Pennsylvania, discusses a study of genetic testing results for patients with metastatic prostate cancer.
The study included cohorts of patients with metastatic prostate cancer in a point-of-care model at Basser Center of Penn Medicine, as well as patients from a cancer genetics practice at the Corporal Michael Crescenz VA Medical Center in Philadelphia. Maxwell and colleagues sought to evaluate the rates of pathogenic germline variants in the context of broader, more diverse patient populations.
Various institutions have implemented different models to streamline genetic testing processes, Maxwell says. Notably, some have integrated nurses into oncology programs to facilitate this testing.
Findings presented at the 2024 AACR Annual Meeting showed that despite differences in the racial breakdowns of each cohort of patients, the prevalence of pathogenic germline variants associated with prostate cancer remained consistent across the cohorts, Maxwell explains. This finding underscores the uniformity of mutation rates across diverse racial groups, challenging previous assumptions regarding racial disparities in genetic mutation prevalence, she continues.
However, difference in rates of variants of uncertain significance were observed, correlating with the racial diversity of each cohort. This suggests that although pathogenic mutation rates appeared to remain consistent, the significance and interpretation of genetic variants may differ among populations, Maxwell explains. Nonetheless, the study underscores the importance of genetic testing for all patients with metastatic prostate cancer and its potential to inform personalized treatment approaches.
By analyzing a cohort of 1000 individuals with metastatic prostate cancer, the study provides a comprehensive population-level perspective on genetic testing, Maxwell says. These data can help enrich the understanding of mutation prevalence beyond traditionally high-risk populations, offering insights into broader demographic representations, she concludes.
Close
A Moment for Men's Health | Inspiring Impact Stories
AT THE BASSER CENTER FOR BRCA, WOMEN’S HEALTH IS ONLY HALF THE STORY
February 24, 2023
Since 2012, the Basser Center for BRCA at Penn Medicine’s Abramson Cancer Center has been a leader in advancing lifesaving therapies and raising crucial awareness of the risks faced by individuals with BRCA gene mutations.
Women with a BRCA1 or BRCA2 mutation have up to a 75% lifetime risk of developing breast cancer, and up to a 50% lifetime risk of developing ovarian cancer.
“While there has been a lot of knowledge and press coverage of the risks of BRCA1 and BRCA2, most has been focused on women,” says Kara Maxwell, Assistant Professor of Medicine at the Perelman School of Medicine. “There is still a lot of misunderstanding and confusion in the general population regarding how men can be affected by a BRCA mutation. It’s critical that we develop new strategies to reach men for genetic counseling and testing and educate them about their risk.”
Fifty percent of the population carrying a BRCA mutation are men. While cancer risks in male BRCA mutation carriers are not as dramatically elevated as those of female BRCA mutation carriers, they still face an increased risk of breast and pancreatic cancer as well as elevated risk of aggressive prostate cancer. Men are significantly less likely to undergo genetic counseling and testing and are often unaware that they have a 50/50 chance of passing this mutation on to each of their children.

Thanks to an anonymous gift, the Basser Center has launched the Men & BRCA program to accelerate research and provide men with specialized BRCA-related care. Maxwell, the Director of the Men & BRCA Program, describes it as “part of a three-pronged approach comprising education, clinical care, and research.”
“The Basser Center is uniquely positioned to accommodate such a program because of the amazing infrastructure that already exists in these three crucial areas,” she says.
Basser has helped to accelerate progress for BRCA-related cancer research, including contributing to the development of FDA-approved targeted therapies for BRCA-related cancers. Before the Center’s establishment, there were no targeted therapies for BRCA cancers. Now, four PARP inhibitors—agents which have been found effective in the treatment of heritable cancers—are available for all four BRCA-related cancer types, thanks in part to research and clinical trials taking place at the Basser Center.

In just six months since receiving a pivotal anonymous gift, the Basser Center has awarded over $350,000 in funding for research projects focused on men’s BRCA-related cancers.

In just six months since receiving a pivotal anonymous gift, the Basser Center has awarded over $350,000 in funding for research projects focused on men’s BRCA-related cancers.

In just six months since receiving a pivotal anonymous gift, the Basser Center has awarded over $350,000 in funding for research projects focused on men’s BRCA-related cancers.

In just six months since receiving a pivotal anonymous gift, the Basser Center has awarded over $350,000 in funding for research projects focused on men’s BRCA-related cancers.
Dennis Holewinski, a BRCA mutation carrier and longtime Basser Center donor, recently took advantage of the opportunity to double his support by tapping into the matching funds made available through the anonymous gift to support a research coordinator who will help expand enrollment on several prostate cancer clinical trials underway at Basser.
“Basser is committed to research that will give families like mine better options to address BRCA-related cancer risk,” says Holewinski. “We’re proud to support this life-saving work and excited to take advantage of matching funds that will allow us to make an even greater impact on research.”
As the Men & BRCA program expands, Maxwell envisions greater potential for helping men with BRCA mutations understand the risks they face and the opportunities available to safeguard their health and the health of their families.
“From a research standpoint there are numerous unanswered questions for men with BRCA1 and BRCA2 mutations,” says Maxwell. “There is a lot of basic science and translational science that we’re interested in pursuing, as well, regarding the interaction of the immune system with the development of cancer.”
The Basser Center hosts many public events and panels to raise awareness and educate audiences about recent developments in the fight against cancer. On March 28, Susan Domchek, Executive Director of the Basser Center, and Robert Vonderheide, Director of the Abramson Cancer Center, will give a talk on Cancer Interception and Prevention as part of the Inspiring Impact Virtual Series. On May 9, Basser’s Breakthroughs & Discoveries Panel will feature leading BRCA expects for a panel discussion focused on advances in screening and treatment for pancreatic cancer, as well as cancer interception and prevention (registration opens in March). Sign up for the Basser Center newsletter to stay up to date on events like this and more news.
Close
Challenge Awards - Class of 2022 | Prostate Cancer Foundation
The PCF Challenge Award Winners – Class of 2022 recipients are:
2022 Movember-PCF VAlor Challenge Award
Principal Investigators: Isla Garraway, MD, PhD (University of California, Los Angeles; Greater Los Angeles VA Healthcare System), Kara Maxwell, MD, PhD (University of Pennsylvania),Corporal Michael J. Crescenz VA Medical Center), Kosj Yamoah, MD, PhD (Moffit Cancer Center), Timothy Rebbeck, PhD (Dana-Farber Cancer Institute; Harvard TH Chan School of Public Health), Brent Rose, MD (University of California, San Diego)
Co-Investigators: Daniel Lee, MD, MS (University of Pennsylvania), Nicholas Nickols, MD, PhD (University of California, Los Angeles), Michael Lewis, MD (Greater Los Angeles VA Healthcare System), Matthew Rettig, MD (University of California, Los Angeles), Saiju Pyarajan, PhD (Brigham and Women’s Hospital)
Project Title: The Rate Elements Skewing Outcomes Linked to Veteran Equity in PCa (RESOLVE PCa) Consortium: Multilevel Modeling to Predict Prostate Cancer Incidence and Aggressiveness
Description:
- Racial disparities in prostate cancer pose a significant public health problem. Understanding the complex factors that contribute to disparities is critical for solving them and improving the lives of patients with prostate cancer.
- In a study in over 70,000 patients with prostate cancer, Dr. Isla Garraway and colleagues found that despite similar screening, self-identified Black/African American patients had a significantly higher incidence of localized and metastatic prostate cancer compared to White/European American patients.
- The team is leveraging a mega-dataset of clinical, demographic, survey and genetic data from over 500,000 Veterans.
- In this project, the team will apply complex modeling approaches to parse out the genetic (polygenic risk score, rare variant alterations, ancestral markers) and non-genetic (self-identified race/ethnicity, social determinants of health, socioeconomic status, and environmental exposures) factors that contribute to disparities in prostate cancer incidence, aggressiveness, and outcomes.
- If successful, this project willdevelop a multi-level prostate cancer risk prediction model and create improved prostate cancer screening and treatment paradigms.
What this means to patients: Understanding the factors that contribute to prostate cancer disparities is a critical unmet medical need. Dr. Garraway and colleagues will use a vast dataset with clinical, demographic, and genetic data from over 500,000 Veterans, to identify social and environmental vs. genomic/genetic factors that contribute to prostate cancer racial disparities and develop models for predicting risk and improving tailored patient screening and management strategies.
Close
2022 Breakthroughs & Discoveries Panel
Close
The Risk of Prostate Cancer in Men with Inherited Germline TP53 Variants - Kara Maxwell
The Risk of Prostate Cancer in Men with Inherited Germline TP53 Variants - Kara Maxwell
March 16, 2022
"In this discussion between Kara Maxwell and Alicia Morgans, Dr Maxwell highlights the role of TP53 in cancer and understanding the Li-Fraumeni syndrome. Inherited germline TP53 pathogenic and likely pathogenic variants (gTP53) cause autosomal dominant multicancer predisposition including Li-Fraumeni syndrome and the work that Drs. Morgans and Maxwell discuss here sought to determine whether gTP53 predisposes to prostate cancer."
Close
Kara N. Maxwell, MD, Ph.D., Assistant Professor of Medicine at Penn Medicine speaks about the Association of Inherited Mutations in DNA Repair Genes with Localized Prostate Cancer.
Close
COVID-19 Vaccinations & Li Fraumeni Syndrome
COVID-19 Vaccinations & Li Fraumeni Syndrome
August 24, 2021
"The LFS Association and Drs. Joshua Schiffman and Kara Maxwell host a special webinar for families with Li‑Fraumeni syndrome to share insights on the COVID‑19 vaccines and concerns within the LFS community.
Guest Panelists Include:
- Joshua Schiffman, MD
Professor of Pediatrics, University of Utah and CEO of PEEL Therapeutics - Kara Maxwell, MD, PhD
Assistant Professor of Medicine, UPenn - Wendy Kohlmann, MS, CGC
Genetic Counselor, Huntsman Cancer Institute, University of Utah - Luke Maese, DO
Assistant Professor of Pediatrics, University of Utah
Panelists address questions and concerns about the coronavirus vaccines and its impact within our community.
Some Questions Answered Include:
- Have there been any reactions from the vaccine on those with LFS, or those who are mosaic?
- Are there any studies or data that support it is safe for an LFS patient to get the vaccine?
- Is there any preferred vaccine for any particular variants of LFS?
- Are booster shots recommended for those with LFS, cancer patients undergoing treatment, cancer patients not undergoing treatment?
- Do those with LFS, yet otherwise very healthy, need to be vaccinated?"
Close
Patients in Cancer Remission Also at Higher Risk for Severe COVID-19 Illness
Patients in Cancer Remission Also at Higher Risk for Severe COVID-19 Illness
January 26, 2021
"Patients with inactive cancer who are not currently undergoing treatment also face a significantly higher risk of severe illness from COVID-19, according to a new study published by Sun et al in JNCI Cancer Spectrum. The findings underscore the importance of COVID-19 mitigation, like social distancing and mask-wearing, and vaccinations for all patients, not just those recently diagnosed or with active disease.
“Patients who have cancer need to be careful not to become exposed during this time,” said senior author Kara N. Maxwell, MD, PhD, Assistant Professor of Hematology-Oncology and Genetics in the Perelman School of Medicine at the University of Pennsylvania and a member of the Abramson Cancer Center and the Basser Center for BRCA. “That message has been out there, but these latest findings show us it’s not only for patients hospitalized or on treatment for their cancer. All oncology patients need to take significant precautions during the pandemic to protect themselves.”
Study Methods
The researchers analyzed the records of more than 4,800 patients who had been tested for COVID-19 from the Penn Medicine BioBank, a centralized bank of samples and linked data from the health system’s electronic health records, to investigate the association between cancer status and COVID-19 outcomes. Of the 328 positive cases through June 2020, 67 (20.7%) had a cancer diagnosis in their medical history (80.6% with solid tumor malignancy and 73.1% with inactive cancer).
Results
Patients with COVID-19—including both those with active cancer (n = 18) and inactive cancer (n = 49)—had higher rates of hospitalizations compared to patients without cancer (55.2% vs 29%), intensive care unit admissions (25.7% vs 11.7%), and 30-day mortality (13.4% vs 1.6%). Although worse outcomes were more likely in patients with active cancer, patients in remission also faced an overall increased risk of more severe disease compared to COVID-19 patients without cancer.
Notably, the proportion of Black patients—who make up 20% of the patients in the biobank—was significantly higher in both cancer and noncancer COVID-19–positive cohorts (65.7% and 64.1%, respectively) compared to all patients tested for SARS-CoV-2. The findings parallel prior reports showing the disproportionate impact of COVID-19 on minority communities.
“We really need to be thinking about race as a significant factor in trying to get people vaccinated as soon as we can,” said Dr. Maxwell.
The study authors concluded, “These results emphasize the critical importance of preventing SARS-CoV-2 exposure and mitigating infection in [patients with] cancer.”"
Close
The Prostate Cancer Foundation Announces 2020 Young Investigator Awards Totaling $6 Million For Prostate Cancer Research
The Prostate Cancer Foundation Announces 2020 Young Investigator Awards
December 16, 2020
"The Prostate Cancer Foundation (PCF) today announced the Class of 2020 Young Investigator Award recipients totaling $6 million in funding for innovative prostate cancer research.
PCF Young Investigator Awards are intended to identify a cohort of future research leaders who will keep the field of prostate cancer research vibrant with new ideas, and offer career and project support for early career physicians and scientists who are committed to advancing the prostate cancer field. Twenty-seven PCF Young Investigator Awards totaling $6 million were granted to the promising next generation of cancer researchers.
PCF Young Investigator Awards – Class of 2020
For full project descriptions visit https://pcf.org/YI2020.
2020 Tad Smith & Caroline Fitzgibbons-PCF Young Investigator Award
Principal Investigator: Juan Arriaga, PhD, Columbia University Medical Center
Mentors: Cory Abate Shen, PhD; Andreas Califano, PhD; Mark Rubin, MD
Project Title: Investigating Novel Epigenetic Drivers of Bone Metastasis and Treatment Response in Metastatic Castration-Resistant Prostate Cancer
2020 Lowell Milken-PCF Young Investigator Award
Principal Investigator: Wayne Brisbane, MD, University of California, Los Angeles
Mentor: Leonard Marks, MD
Project Title: Micro-Ultrasound and MRI Investigation of Human Prostate Cancer
2020 Larry Ruvo-PCF Young Investigator Award
Principal Investigator: Jeremie Calais, MD, University of California, Los Angeles
Mentors: Johannes Czernin, MD; Matt Rettig, MD; Robert Reiter, MD
Project Title: Validating a PET Imaging Biomarker for Targeting Fibroblast Activation Protein in Prostate Cancer Stroma
2020 James Maguire-PCF VAlor Young Investigator Award
Principal Investigator: Lisa Chesner, PhD, University of California, San Francisco
Mentors: Felix Feng, MD; Lawrence Fong, MD; Matthew Cooperberg, MD, MPH
Project Title: Understanding the Role of Androgen Signaling in Facilitating Immune Evasion in Advanced Prostate Cancer
2020 Rob & Cindy Citrone-PCF Young Investigator Award
Principal Investigator: Jonathan Chou, MD, PhD, University of California, San Francisco
Mentors: Alan Ashworth, PhD; Felix Feng, MD
Project Title: Discovering Precision Oncology Approaches for CDK12-Deficient Prostate Cancer
2020 Tad Smith & Caroline Fitzgibbons-PCF Young Investigator Award
Principal Investigator: Momeneh (Sepideh) Foroutan, PhD, Monash University
Mentors: Shahneen Sandhu, MBBS; Nicholas Huntington, PhD; Joseph Cursons, PhD
Project Title: Defining the Role of Natural Killer Cells in the Radiotherapy Treatment Response of Metastatic Prostate Cancer
2020 CRIS Cancer Foundation-PCF Young Investigator Award
Principal Investigator: Francesco Giganti, MD, University College London
Mentors: Caroline M. Moore, MD; Monique Roobol-Bouts, PhD
Project Title: A Multi-Center Study to Assess the Impact of MRI for Detection of Aggressive Prostate Cancer in Men on Active Surveillance
2020 Tad Smith & Caroline Fitzgibbons-PCF Young Investigator Award
Principal Investigator: Rebecca Graff, ScD, University of California, San Francisco
Mentors: June Chan, ScD; Peter Carroll, MD, MPH; John Witte, PhD
Project Title: Exploration of Metabolomics for the Prevention of Lethal Prostate Cancer
2020 Tad Smith & Caroline Fitzgibbons-PCF Young Investigator Award
Principal Investigator: Sachin Kumar Gupta, PhD, Baylor College of Medicine
Mentor: Laising Yen, PhD
Project Title: Investigating the Pathological Role of AZI1 RNA in TMPRSS2-ERG Gene Fusion Formation
2020 Neil & Sandra DeFeo Family Foundation-PCF Young Investigator Award
Principal Investigator: Jessica Hawley, MD, Columbia University Medical Center
Mentor: Charles Drake, MD, PhD
Project Title: Association of Circulating Markers of Pro-Tumorigenic Inflammation with Clinical Progression and Race-Ethnicity in Men with Prostate Cancer
2020 CRIS Cancer Foundation-PCF Young Investigator Award
Principal Investigator: Anastasia Hepburn, PhD, Newcastle University
Mentors: Rakesh Heer, PhD; Robert Bristow, MD
Project Title: Improving Outcomes for PARP Inhibition Treatment in Men with Lethal BRCA2 Mutant Prostate Cancer by Targeting the Tumor Microenvironment
2020 Foundation Medicine-PCF Young Investigator Award
Principal Investigator: Daniel Khalaf, MD, BC Cancer Agency
Mentors: Kim Chi, MD; Alexander Wyatt, PhD
Project Title: Developing a Novel ctDNA-Based Approach to Patient Risk Stratification and Treatment Selection in mCRPC: A Large Population-Cased Cohort Study
2020 Peter & Laurie Grauer-PCF Young Investigator Award
Principal Investigator: Vadim Koshkin, MD, University of California, San Francisco
Mentors: Eric Small, MD; Thomas Hope, MD; Luke Gilbert, PhD
Project Title: Using CDK4/6 Inhibition to Augment PSMA Expression in Advanced Prostate Cancer and Enhance Clinical Responses to PSMA-Targeted Radioligand Therapy
2020 Rebecca and Nathan Milikowsky-PCF Young Investigator Award
Principal Investigator: Ariel Marciscano, MD, Weill Cornell Medicine
Mentors: Chris Barbieri, MD, PhD; Sandra Demaria, MD; Charles Drake, MD, PhD
Project Title: Targeting Fc Gamma Receptors with Stereotactic Radiation to Reprogram Myeloid-Derived Immune Cells in the Prostate Tumor Microenvironment
2020 Gary and Allison Lieberman-PCF VAlor Young Investigator Award
Principal Investigator: Kara Maxwell, MD, PhD, Corporal Michael J. Crescenz VA Medical Center/University of Pennsylvania
Mentors: Naomi Haas, MD; Kyle Robinson, MD
Project Title: Towards Targeting African American Prostate Cancer with PARP Inhibitors and Immunotherapy
2020 Tad Smith & Caroline Fitzgibbons-PCF Young Investigator Award
Principal Investigator: Lucia Nappi, MD, PhD, University of British Columbia
Mentors: Martin Gleave, MD; Amina Zoubeidi, PhD
Project Title: Uncovering Lineage Plasticity in the Context of Specific Genomic Alterations in High Risk Prostate Cancer
2020 David Yurman-PCF VAlor Young Investigator Award
Principal Investigator: Ravi Parikh, MD, Corporal Michael J. Crescenz VA Medical Center/University of Pennsylvania
Mentors: Alicia Morgans, MD, MPH; Ravishankar Jayadevappa, PhD
Project Title: Biomarker-Based Approaches to Predict Fracture Risk among Men with Metastatic Hormone-Sensitive Prostate Cancer
2020 The Kovler Family Foundation-PCF Young Investigator Award
Principal Investigator: Jung Wook Park, PhD, Duke University
Mentors: Jiaoti Huang, MD, PhD; Andrew Armstrong, MD
Project Title: Interrupting the Aberrant Cancer Development Sequence in Prostate Cancer Progression
2020 John Black Charitable Foundation-PCF Young Investigator Award
Principal Investigator: Alec Paschalis, MD, PhD, Institute of Cancer Research
Mentors: Johann de Bono, MD, PhD; Stephen Plymate, MD; Ganesh Raj, MD, PhD
Project Title: Metabolic Adaptations to Androgen Receptor Blockade and the Prostate Cancer Transcriptome
2020 Jeff & Loyd Zisk-PCF Young Investigator Award
Principal Investigator: Antonio Rodriguez-Calero, MD, University of Bern
Mentors: Mark Rubin, MD; Savatore Piscuoglio, PhD
Project Title: Molecular Pathology-Artificial Intelligence Approach to Therapy Response Prediction for Castration Resistant Prostate Cancer
2020 ASTRO-PCF Early Career Development Award to End Prostate Cancer-Richard and Ellen Sandler YoungInvestigator Award
Principal Investigator: Tyler Seibert, MD, PhD, University of California, San Diego
Mentors: Anders Dale, PhD; Michael Hahn, MD, PhD; Loren Mell, MD
Project Title: Phase II Biomarker Study of Advanced Diffusion MRI in High-Risk, Localized Prostate Cancer Treated with Radiotherapy and Androgen Deprivation Therapy
2020 Advanced Accelerated Applications-PCF Young Investigator Award
Principal Investigator: Alok Tewari, MD, PhD, Harvard University/Dana-Farber Cancer Institute
Mentors: Eliezer Van Allen, MD; Myles Brown, MD; Mary-Ellen Taplin, MD
Project Title: Integrated Single-Cell Analysis of Mechanisms of Therapeutic Resistance in Prostate Cancer Patients
2020 Michael & Patricia Berns-PCF Young Investigator Award
Principal Investigator: Jeffrey Tosoian, MD, University of Michigan
Mentors: Arul Chinnaiyan, MD, PhD; Todd Morgan, MD; Bruce Trock, PhD
Project Title: Refinement, Validation and Clinical Application of a Novel Panel of High-Grade Cancer-Specific Biomarkers in the Overall and African American Populations
2020 Emilio Bassini-PCF Young Investigator Award
Principal Investigator: Elizabeth Wasmuth, PhD, Memorial Sloan Kettering Cancer Center
Mentors: Charles Sawyers, MD; Sebastian Klinge, PhD
Project Title: Biochemical, Structural and Molecular Dissection of Androgen Receptor Transcriptional Activity
2020 The Boehly Family-PCF Young Investigator Award
Principal Investigator: Leanne Woods-Burnham, PhD, City of Hope
Mentors: Rick Kittles, PhD; Tanya Dorff, MD
Project Title: HER2 Expression in African American Men with Prostate Cancer
2020 Ms. Lucy Shostak & Dr. Elliot Abramowitz-PCF Young Investigator Award
Principal Investigator: Samir Zaidi, MD, PhD, Memorial Sloan Kettering Cancer Center
Mentors: Charles Sawyers, MD; Michael Morris, MD
Project Title: Studying the Mechanisms of Lineage Plasticity in Prostate Cancer
2020 Tad Smith & Caroline Fitzgibbons-PCF Young Investigator Award
Principal Investigator: Jimmy Zhao, MD, PhD, Memorial Sloan Kettering Cancer Center
Mentor: Charles Sawyers, MD
Project Title: Investigating Cell of Origin and Molecular Mechanisms of Lineage Plasticity in Neuroendocrine Prostate Cancer"
Close
Reviewing Various Biomarker Settings for Patients with Prostate Cancer
Reviewing Various Biomarker Settings for Patients with Prostate Cancer
December 4, 2020
"The last 5 years in prostate cancer have seen exponential growth for the field of biomarkers. Specifically, not only do guidelines that now incorporate many biomarkers offer guidance on how to treat these patients, but they can also assess the potential for developing prostate cancer.
Kara N. Maxwell, MD, PhD, expanded upon the available biomarkers for use in prostate cancer in a presentation during the 21st Annual Meeting of the Society of Urologic Oncology (SUO). She explained how the available biomarkers can be applied at various points, while also guiding toward which treatments can improve patient care.
Maxwell, an assistant professor in the Departments of Medicine-Hematology/Oncology and Genetics, Penn Medicine, explained that with any type of cancer, biomarkers can fall into several different categories: biomarkers of risk, diagnostic biomarkers, and prognostic and predictive biomarkers for treatment. “Many of these categories are starting to blend [in prostate cancer],” she commented.
“Things have gotten very confusing in the biomarker space when we think about genomics, and it's very important when you're thinking about treating your patient with one of these therapies to think carefully about the test that you’ve ordered.”
Risk Biomarkers
Risk biomarkers entail germline genetic factors that predetermine that a man is at risk for developing prostate cancer. Maxwell explained that these mutations range from common single nucleotide polymorphisms that do not carry a significant risk of cancer to the more rare but traditional cancer genes, such as BRCA1/2, that are associated with a more significantly increased risk of cancer. She noted that HOXB13 G84E is also associated with a 2 to 3 times increased risk of prostate cancer.
Several of these risk biomarkers have prognostic and predictive value as well, including BRCA2.
“Things have really expanded and changed in the germline world with a number of different companies offering tests. And really [this] is to make the point that…as a practicing clinician, one cannot assume what genes your patients have been tested for unless you actually see the test that they underwent,” she commented.
Diagnostic Biomarkers
Diagnostic biomarkers in prostate cancer typically include prostate-specific antigen (PSA) level testing, but testing is also ongoing for several urine and tissue-based biomarkers. These biomarkers can also potentially be used in treatment monitoring, and the prognostic value of these markers continues to be explored.
Prognostic/Predictive Biomarkers
Maxwell explained that since a landmark article was published in Cell in 2015 defining many of the genomic alterations commonly found in metastatic prostate cancer,2 many biomarkers have been more defined in prostate cancer and incorporated into guidelines for recommended genomic testing. Most research since then has focused on alterations in DNA repair mechanisms that can be targeted with genomically-targeted therapies, like PARP inhibitors.
Olaparib (Lynparza) is an FDA-approved PARP inhibitor for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) who harbor deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene mutations and have progressed on prior treatment with enzalutamide (Xtandi) or abiraterone acetate (Zytiga). The approval was based on findings from the phase 3 PROfound trial (NCT02987543).3
The companion diagnostics approved for use with olaparib are the FoundationOne CDx for somatic mutations and the Myriad BRACAnalysis CDx for germline mutations. This Myriad test also evaluated homologous recombination deficiency in patients who are negative for germline mutations.
In the trial, patients were tested for HRR gene mutations for inclusion in the study of BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, and RAD54L using the FoundationOne CDx assay. In PROfound, responses did vary according to the gene alterations, with patients with BRCA1 (HR, 0.41) and BRCA2 (HR, 0.21) showing significant benefit from olaparib whereas those with PPP2R2A did not demonstrate a benefit from treatment with the PARP inhibitor (HR, 6.61).
“It is important to note that this is somatic-only testing and does not distinguish whether or not these patients carry germline or somatic mutations, and notably, also did not distinguish whether or not they may carry mutations in their lymphocytes by a process known as clonal hematopoiesis of indeterminate potential [CHIP],” Maxwell said. “However, going with the assumption that most of these are either tumor or germline mutations, the PROfound study did show significant benefit of olaparib in BRCA-mutated patients.”
Rucaparib (Rubraca) has a different indication; it is approved for the treatment of patients with deleterious BRCA mutation associated mCRPC, whether germline and/or somatic, based on findings from the TRITON2 trial.4
Although responses were mostly seen with rucaparib in patients with BRCA mutations, additional analyses have explored responses in patients with other HRR mutations tested in the trial (BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK2, FANCA, NBN, PALB2, RAD51, RAD51B, RAD51C, RAD51D, and RAD54L).In the analysis, limited responses were observed in patients with ATM, CDK12, and CHEK2 alterations, including a radiographic response rate of 10.5% in the ATM cohort.Responses were also observed in patients with DNA damage repair gene alterations with a radiographic response rate of 28.6%.5
Maxwell stated, “the question you want to ask yourself is I have a [patient with] metastatic prostate cancer in front of me, what tests do I want to order that will evaluate these 2 very promising, and both FDA approved, therapies for my patients?”
The FoundationOne CDx is one of the more commonly ordered tests in the Northeast and covers all of the HRR genes included in the FDA approval for olaparib among the 309 genes tested with the assay. The assay also covers microsatellite instability (MSI) testing. Maxwell clarified that the FoundationOne Liquid assay does not cover all of the HRR alterations.
Additional biomarkers for treatment in prostate cancer include biomarkers that relate to tumor-agnostic approvals, including MSI-high disease for use with pembrolizumab (Keytruda) or NTRK gene fusions for use with either larotrectinib (Vitrakvi) or entrectinib (Rozlytrek).
NTRK gene fusions are found infrequently in prostate cancers, with NTRK1 demonstrating a frequency of 3.0% (2 of 67) and NTRK3 of 1.5% (1 of 67) in an analysis of actionable genomic alterations in prostate cancer.6 However, when these alterations are found, both TRK inhibitors can be beneficial in this setting.
When pembrolizumab was tested in patients with prostate cancer in the KEYNOTE-199 trial (NCT02787005), responses to treatment were limited; however, this was not a biomarker-based study so it did include all-comer patients with mCRPC. The response rate in KEYNOTE-199 was 3% to 5%.7
However, an analysis of a few patients with metastatic prostate cancer and MSI-high disease, as detected by circulating tumor DNA with the Guardant360 assay, showed a higher response rate of 60%, including 1 complete response.8 Additionally, about half of the patients had both MSI-high disease and alterations in BRCA1/2.
References:
1. Maxwell KN. Biomarker Review: Predictive/Actionable Biomarkers for Metastatic Prostate Cancer. Presented at: 21st Annual Meeting of the Society of Urologic Oncology; December 2-5, 2020; Virtual.
2. Dan R, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
4. Abida W, Patnaik A, Campbell D, et al; TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. Published online August 14, 2020. doi:10.1200/JCO.20.01035
5. Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res. 2020;26(11):2487-2496. doi:10.1158/1078-0432.CCR-20-0394
6. Ikeda S, Elkin SK, Tomson BN, Carter JL, Zurzrock R. Next-generation sequencing of prostate cancer: genomic and pathway alterations, potential actionability patterns, and relative rate of use of clinical-grade testing. Cancer Biol Ther. 2019;20(2):219-226. doi:10.1080/15384047.2018.1523849
7. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol. 2020;38(5):395-405. doi:10.1200/JCO.19.01638
8. Barata P, Agarwal N, Nussenzveig R, et al. Clinical activity of pembrolizumab in metastatic prostate cancer with microsatellite instability high (MSI-H) detected by circulating tumor DNA. J Immunother Cancer. 2020;8(2):e001065. doi:10.1136/jitc-2020-001065"
Close
Rare TP53 Mutation Confers Risk of Several Cancers in Ashkenazi Jewish Population
Rare TP53 Mutation Confers Risk of Several Cancers in Ashkenazi Jewish Population
July 28, 2020
"A lower-risk TP53 mutation has been linked with a specific kind of Li Fraumeni Syndrome (LFS) which predisposes individuals to a wide range of cancers, and this newly described variant of p53 is most commonly found in the Ashkenazi Jewish (AJ) population, according to results from a study recently published in Cancer Research.1
“We identified a rare TP53 tetramerization domain missense mutation, c.1000G>C;p.G334R, in a family with multiple late-onset LFS-spectrum cancers,” the authors wrote. “Twenty additional c.1000G>C probands and one c.1000G>A probrand were identified, and available tumors showed biallelic somatic inactivation of TP53. The majority of families were of Ashkenazi Jewish descent, and the TP53 c.1000G>C allele was found on a commonly inherited Chromosome 17p13.1 haplotype.”
Mutations in the TP53 are the most commonly acquired mutations in cancer; when these mutations are inherited, they can lead to LFS, which is a syndrome that puts individuals at a 90% likelihood of developing cancers, such as soft tissue and bone sarcomas, breast and brain cancers, adrenocortical tumors, and leukemia, at some point in their lifetime.2
In the study, investigators performed whole exome sequencing on several members of 8 different families. Results revealed TP53 c.1000G>C;p.G334R in a pair of third-degree relatives of a family with several LFS-component cancers which were mostly identified in the fourth to ninth decades, in addition to 5 family members with several primary malignancies.
Seven additional independent families with c.1000G>C were discovered via clinical genetics practices, according to the study authors. One such family demonstrated segregation of LFS-component cancers to third-degree relatives. Although the majority of affected individuals were found to have adult-onset cancers, 6 individuals from 4 different families were noted to have adrenocortical tumors (ACTs). A total of 8 individuals from 4 families were found to have multiple primary tumors.
Two genetic testing laboratory cohorts and literature review identified 13 additional TP53 c.1000G>C;p.G334R carriers. Among 309,922 patients undergoing testing at Ambry Genetics Laboratories, 8 probands were identified; 3 probands were identified among 21,729 patients who underwent tumor genomic profiling at Memorial Sloan Kettering Cancer Center. A total of 3 probands were found to be unaffected although they came from multicancer families. Eleven probands were found to have several tumor types in the fifth to eighth decades; 2 of these had multiple primary tumors.
For 16 probands with TP53 c.1000G>C, p.G334R, ancestry data were available. Results showed that all 8 families in the clinical cohort and 7 of 8 families from the 2 genetic testing cohorts, had AJ ancestry. “The mutation was found at an approximately ten-fold enrichment in AJ versus non-AJ individuals in both genetic testing cohorts (0.023% AJ vs 0.001% of Caucasian non-AJ and 0.07% AJ vs 0.005% of non-AJ,” the study authors wrote. Additional analyses revealed that 9 individuals from 4 families all had the chromosome 17p13.1 haplotype.
When combining all the data collected, investigators found that the newly identified mutation presents a risk of childhood cancer in certain families, but later onset cancers in other families, with a lifetime risk lower than 90%, according to the press release. After investigators from St. Jude Children’s Research Hospital modeled the TP53 mutation to determine its impact on the protein’s structure, investigators revealed an “inherited set of genetic material” that was present in all individuals harboring the mutation. These finding suggested that the newly discovered mutation was predominantly observed in 1 ethnicity: the AJ population.
Subsequently, an investigational team from The Wistar Institute found that the mutation impacted the p53 protein in cells and affected the expression of p53 target genes, suggesting that the mutation may be a key player in transformation and cancer formation. As no therapies targeting the p53 pathway are currently available, investigators are currently examining how this condition might be targeted and treated.
“Due to the wide variety of disease types associated with inherited TP53 mutations and the early age of cancer diagnoses, cancer screening is exceptionally aggressive,” Kara N. Maxwell, MD, PhD, a senior researcher on the study, an assistant professor of Hematology-Oncology and Genetics in the Perelman School of Medicine at the University of Pennsylvania, and a member of the Abramson Cancer Center and the Basser Center for BRCA, stated in a press release. “However, we do not yet know if all mutations require the same high level of screening. It is therefore critical to study the specifics of individual TP53 mutations so we can understand how best to screen people who carry lower risk mutations.”
As this population of patients seem to have a different risk profile compared with patients with classic LFS, investigators have begun to question whether the standard approach utilizing full-body scans should be modified. To this end, investigators are developing liquid biopsy techniques that may help to improve detection within this patient population.
“By identifying and understanding this Ashkenazi variant of p53, our goal is to help people who have genetic variants of this critical gene to better understand their cancer risk, and eventually to assist the development of new specific treatments that will reduce the burden of cancer on this population,” Maureen E. Murphy, PhD, Ira Brind professor and program leader of the Molecular and Cellular Oncogenesis Program of the Wistar Cancer Center stated in the press release.
References
- Powers J, Pinto EM, Barnoud T, et al. A rare TP53 mutation predominant in Ashkenazi Jews confers risk of multiple cancers. Cancer Res. Published online July 16, 2020. doi:10.1158/0008-5472.CAN-20-1390
- Rare mutation of TP53 gene leaves people at higher risk for multiple cancers. News release. Penn Medicine. July 16, 2020. Accessed July 28, 2020. https://bit.ly/30V7IAX."
Close
Rare genetic mutation leaves people at higher risk for multiple cancers
Rare genetic mutation leaves people at higher risk for multiple cancers
July 20, 2020
"Rare inherited mutations in the body’s master regulator of the DNA repair system—the TP53 gene—can leave people at a higher risk of developing multiple types of cancer over the course of their lives. Now, for the first time, a team led by researchers in the Basser Center for BRCA at the Abramson Cancer Center details the potential implications of a lower risk TP53 mutation, including an association with a specific type of Li-Fraumeni syndrome (LFS), an inherited predisposition to a wide range of cancers. The findings raise questions about how to appropriately screen patients for this mutation and whether the standard process of full-body scans for LFS patients should be modified for this group, since their risk profile is different than those with classic LFS. The researchers published their findings in Cancer Research, a journal of the American Association for Cancer Research.
Mutations in the TP53 gene are the most commonly acquired mutations in cancer. The p53 protein, made by the TP53 gene, normally acts as the supervisor in the cell as the body tries to repair damaged DNA. Different mutations can determine how well or how poorly that supervisor is able to direct the response. The more defective the mutation, the greater the risk. When TP53 mutations are inherited, they cause LFS, a disease that leaves people with a 90 percent chance of developing cancer in their lifetime. There are currently no therapies that target the p53 pathway.
Researchers determined that there is an inherited set of genetic material shared among people who have this mutation, suggesting it’s what’s called a founder mutation—a mutation that tracks within one ethnicity. In this case, that ethnicity is the Ashkenazi Jewish population.
“Due to the wide variety of disease types associated with inherited TP53 mutations and the early age of cancer diagnoses, cancer screening is exceptionally aggressive. However, we do not yet know if all mutations require the same high level of screening,” says the study’s senior author Kara N. Maxwell, an assistant professor of hematology-oncology and Genetics in the Perelman School of Medicine and a member of the Abramson Cancer Center and the Basser Center for BRCA. “It is therefore critical to study the specifics of individual TP53 mutations so we can understand how best to screen people who carry lower risk mutations.”"
Close
News release on our latest publication about Ashkenazi Jewish founder TP53 mutation associated with Li-Fraumeni Syndrome (LFS)
July 16, 2020
Rare Mutation of TP53 Gene Leaves People at Higher Risk for Multiple Cancers
"Rare inherited mutations in the body’s master regulator of the DNA repair system – the TP53 gene – can leave people at a higher risk of developing multiple types of cancer over the course of their lives. Now, for the first time, a team led by researchers in the Basser Center for BRCA at the Abramson Cancer Center of the University of Pennsylvania details the potential implications of a lower risk TP53 mutation, including an association with a specific type of Li-Fraumeni syndrome (LFS), an inherited predisposition to a wide range of cancers. The researchers published their findings today in Cancer Research, a journal of the American Association for Cancer Research.
Mutations in the TP53 gene are the most commonly acquired mutations in cancer. The p53 protein, made by the TP53 gene, normally acts as the supervisor in the cell as the body tries to repair damaged DNA. Different mutations can determine how well or how poorly that supervisor is able to direct the response. The more defective the mutation, the greater the risk. When TP53 mutations are inherited, they cause LFS, a disease that leaves people with a 90 percent chance of developing cancer in their lifetime. These commonly include soft tissue and bone sarcomas, breast and brain cancer, adrenocortical tumors, and leukemia, and patients undergo frequent screening starting as infants to look for signs of disease, given the high risk of childhood cancers that continues throughout their lives. There are currently no therapies that target the p53 pathway.
“Due to the wide variety of disease types associated with inherited TP53 mutations and the early age of cancer diagnoses, cancer screening is exceptionally aggressive. However we do not yet know if all mutations require the same high level of screening,” said the study’s senior author Kara N. Maxwell, MD, PhD, an assistant professor of Hematology-Oncology and Genetics in the Perelman School of Medicine at the University of Pennsylvania and a member of the Abramson Cancer Center and the Basser Center for BRCA. “It is therefore critical to study the specifics of individual TP53 mutations so we can understand how best to screen people who carry lower risk mutations.”
The study’s co-first authors are Jacquelyn Powers, MS, LCGC, a genetic counselor in the Basser Center for BRCA, and Emilia Modolo Pinto, PhD, an associate scientist at St. Jude Children's Research Hospital.
For this study, researchers genetically sequenced multiple members of eight different families, then combined those data into a study cohort, along with data of carriers from two genetic testing cohorts. They found this newly identified mutation confers a risk of childhood cancer in some families, but only later onset cancers in others, and likely at a lower than 90 percent lifetime risk.
The St. Jude team, as well as researchers from The Wistar Institute, were critical to the next stage of the research.
The St. Jude team modeled the TP53 mutation to try to figure out what effect it had on the protein’s structure. In addition, working closely with the Penn team, the St. Jude team also determined that there is an inherited set of genetic material shared among people who have this mutation, suggesting it’s what’s called a founder mutation – a mutation that tracks within one ethnicity. In this case, that ethnicity is the Ashkenazi Jewish population.
“By using the same model that led us to determine a founder mutation widespread in Brazilian individuals we have determined this new one in the Ashkenazi Jewish population,” Pinto said.
A team from The Wistar Institute studied the consequences of the new mutation on the function of the p53 protein in cells and showed it affects the expression levels of multiple p53 target genes, suggesting this might play a role in transformation and cancer formation.
“By identifying and understanding this Ashkenazi variant of p53, our goal is to help people who have genetic variants of this critical gene to better understand their cancer risk, and eventually to assist the development of new specific treatments that will reduce the burden of cancer on this population,” said Maureen E. Murphy, PhD, Ira Brind Professor and program leader of the Molecular & Cellular Oncogenesis Program of the Wistar Cancer Center.
The findings raise questions about how to appropriately screen patients for this mutation and whether the standard process of full-body scans for LFS patients should be modified for this group, since their risk profile is different than those with classic LFS.
Maxwell and her team are part of a group working on developing liquid biopsy techniques – a blood test that would be able to improve detection. They say they’re hopeful this study will help inform future liquid biopsy work.
This study was supported by the National Institutes of Health Grants (K08CA215312, P30CA016520, R01CA102184, K99CA241367, K08CA234394, KL2TR00187903, R01CA242218, RC4CA153828, R01CA225662), the Basser Center for BRCA, the Breast Cancer Research Foundation, the Burroughs Wellcome Foundation, the International Pediatric Adrenocortical Tumor Registry, and ALSAC – the fundraising and awareness organization of St. Jude."
Close
Li-Fraumeni Syndrome & COVID-19 Webinar
Li-Fraumeni Syndrome & COVID-19 Webinar
Friday, March 27, 2020
"The LFS Association and Dr. Joshua Schiffman from the Huntsman Cancer Institute host a special webinar for families with Li-Fraumeni syndrome to share insights on the COVID-19 virus and how it affects the LFS community.
Guest Panelists Include:
Joshua Schiffman, MD, Professor of Pediatrics, University of Utah and CEO of PEEL Therapeutics
Luke Maese, DO, Assistant Professor of Pediatrics, University of Utah
Wendy Kohlmann, MS, CGC, Genetic Counselor, Huntsman Cancer Institute, University of Utah
Jennie Vagher, CGC, Genetic Counselor, Huntsman Cancer Institute, University of Utah
Kara Maxwell, MD, PhD, Assistant Professor of Medicine, UPenn
Panelists address questions and concerns about the coronavirus and its impact within our community."
Close
Progress and Promise Against Cancer - American Association for Cancer Research (AACR)
Progress and Promise Against Cancer
"Join the Conversation:
Ask Your Cancer Research Question
Share Your Cancer Story
Ask a Question or Share a Comment Here
Text to 844-432-AACR
Tweet #PhillyTalksCancer
Today on NBC10 and Telemundo 62 from 11 a.m. to 6:30 p.m., the American Association for Cancer Research (AACR) has brought together distinguished AACR members from the Philadelphia region to answer your questions. Ask a cancer research question spanning prevention, early detection and diagnosis, treatment, and survivorship. Cancer survivor advocates are also in-studio to learn about your cancer journey, and share their own.
If you would like to ask a cancer research question or have a comment, please fill out the form below. An expert or advocate will respond during the day."
Close
"The Reinterpretation of Genetic Tests Is Rare" by Kara Maxwell, MD, PhD - An opinion article published in WSJ
The Reinterpretation of Genetic Tests Is Rare
Jan. 8, 2020 2:06 pm ET
“Gene Tests Led to Surgery, Then the Odds Changed” (Page One, Dec. 21) describes a family’s experience with genetic testing, which included a rare event: a reinterpretation of genetic test results from “likely harmful” to “uncertain.” It is important to emphasize that it is extremely rare that genetic testing reports change an interpretation from harmful to anything less than that. Individuals who have been told that they have a BRCA1 or BRCA2 mutation should know that it is exceedingly unlikely that anything has changed. In the rare event that the interpretation of genetic testing results change from harmful to uncertain (or vice versa), the health-care provider who ordered the test is notified. It’s important for individuals who have undergone genetic testing to keep their contact information current with their health-care providers.
Genetics is a field of risks—not absolutes—and specific answers for a specific patient are nuanced and can be difficult. We understand that the implications of the recommendations we provide are life-changing. Decisions about options and timing of preventive surgery and other strategies are very personal. Since the cloning of BRCA1 and BRCA2 25 years ago, there have been tremendous advances. Research must continue to advance so we can provide even more precise and lifesaving guidance.
Kara Maxwell, M.D., Ph.D.
Close
Prostate Cancer Screening with a BRCA Mutation
Prostate Cancer Screening with a BRCA Mutation
"This is part of an ongoing series featuring interviews with physicians on topics related to hereditary cancer. This article was written by Kara Maxwell, MD, PhD. Dr. Maxwell is a physician scientist with the Basser Center and the Abramson Cancer Center at the University of Pennsylvania. Her areas of expertise are in cancer genetics, specifically in hereditary cancer syndromes."
Close
A New Recommendation Adds To The List Of Drugs That Can Lower Breast Cancer Risk For Women With High Risk Of Developing The Disease
A New Recommendation Adds To The List Of Drugs That Can Lower Breast Cancer Risk For Women With High Risk Of Developing The Disease
September 11, 2019
"The recommendation also said that women who are not at high risk should not consider taking these medications, because the side effects of the medications will likely outweigh the benefits.
“I am in strong agreement with the recent recommendations by the USPSTF regarding the use of tamoxifen, raloxifene and aromatase inhibitors for breast cancer risk reduction in women at high risk for breast cancer,” Dr. Kara Maxwell in the Basser Center for BRCA at Penn’s Abramson Cancer Center tells SurvivorNet.
The recommendation draws on findings from more than 5 million women, who participated in 46 studies. were associated with lower rates of breast cancer in women with a high risk of developing the disease.
Doctors are able to make some assessments of risk for breast cancer based on a few observable factors, “We perform a personalized risk-benefit analysis on all women seen in our cancer risk evaluation clinic that incorporates family history, breast density, reproductive history and prior benign breast biopsy results. We routinely recommend medications to reduce breast cancer risk for women at high risk of breast cancer,” says Dr. Maxwell.
But there are still some problems with determining which women fall into the “high-risk” category, because many women who don’t have easily detectable genetic mutations may still be at risk.
“The major outstanding issue in the field, however, is the need for better individualized breast cancer risk assessment tools in women without BRCA1/2 mutations to more accurately identify those women who truly are in the “high risk category,” says Dr. Maxwell. “For example, models are needed using 10-year risk instead of lifetime risk and those incorporating low to moderate risk genetic variants.”"
Close
Pictures




Close
American Society of Human Genetics ASHG 2018 Annual Meeting
Close
Top Physician-Scientists Receive $8.4 Million Award From Burroughs Wellcome Fund
Top Physician-Scientists Receive $8.4 Million Award From Burroughs Wellcome Fund
May 23, 2017
"Twelve talented medical doctors at some of America’s top health research institutions are recipients of career development grant from the Burroughs Wellcome Fund — the private, independent foundation making personal investments in biomedical research and careers for more than 60 years.
The 2017 recipients of the BWF Career Awards for Medical Scientists (CAMS) include physician-scientists from Baylor College of Medicine, Harvard Medical School, University of California-Los Angeles, University of California-San Diego, University of Pennsylvania, University of Texas Southwestern Medical Center-Dallas, University of Washington, and Washington University.
“The declining population of physician scientists has been an area of focus for the Burroughs Wellcome Fund for the past decade,” said Burroughs Wellcome Fund President John Burris. “The complexity of the physician scientist career path has increased as biomedical science has evolved. Our funding provides dollars for research as well as for protecting time spent for conducting research.”
The CAMS program provides $700,000 in funding over five years, to help physicians transition into a full-time career as a biomedical research scientists and tenured faculty members. Including the 2017 cohort, CAMS have been awarded to 122 physician-scientists over the past 10 years.
The 2017 CAMS Awardees and their Research Focus
Vijay Garud Bhoj, M.D., Ph.D.
University of Pennsylvania
Development of CAR T-cell immunotherapy for prevention and eradication of FVIII inhibitors in Hemophilia A
Lindsay Catherine Burrage, M.D., Ph.D.
Baylor College of Medicine
Impaired glycogen metabolism and chronic liver disease in urea cycle disorders
Aaron Foster Carlin, M.D., Ph.D.
University of California-San Diego
Deciphering human innate immune responses to Zika virus infection
Alejandro Chavez, M.D., Ph.D.
Harvard Medical School
Novel technologies and their application to neurodegenerative diseases
Whitney Elizabeth Harrington, M.D., Ph.D.
University of Washington
Defining the role of maternal cells in fetal and infant immunity to malaria
Tamia Alisha Harris-Tryon, M.D., Ph.D.
University of Texas Southwestern Medical Center-Dallas
The Function of Resistin Like Molecule alpha (RELMalpha) in Cutaneous Host Defense
Kara Noelle Maxwell, M.D., Ph.D.
University of Pennsylvania
A genotype-phenotype study of tumors from patients with inherited mutations in DNA repair genes
Kent William Mouw, M.D., Ph.D.
Harvard Medical School
Investigating the effect of ERCC2 mutations on DNA repair capacity and chemo-radiotherapy response in muscle-invasive bladder cancer
Anoop Patel, M.D.
University of Washington
Deep interrogation and modeling of intratumoral heterogeneity, plasticity, and tumor evolution in glioblastoma
Tamer Sallam, M.D., Ph.D.
University of California-Los Angeles
Spatial control of nuclear receptor regulatory circuits in cardiovascular disease
Zuzana Tothova, M.D., Ph.D.
Harvard Medical School
Elucidating the mechanisms of cohesinopathy in myelodysplastic syndromes
Craig Brian Wilen, M.D., Ph.D.
Washington University
Role of virus-receptor interactions in determining norovirus tropism and pathogenesis"
Close
2016 Basser Center Symposium Interview
Close
Breast Cancer Clinical Trials
Close
Early Stage Breast Cancer
Close