Projects
Pathological cardiac hypertrophy induced by increased biomechanical stress is a fundamental mechanism in the development of myocardial hypertrophy and heart failure (HF) while biomechanical unloading often induces significant regression of myocardial pathology. We have developed novel biomaterials that allow bi-directional control of substrate stiffness that myocardial cells “feel” and have employed these materials to both two- (2D) and three-dimensional (3D) culture formats. In conventional 2D culture formats, the high degree of temporal control provided by these new tunable elastomers are proving ideal for studies of “mechanical memory": persistent cellular remodeling following a transient biomechanical stimulus. Recognizing shortcomings of 2D formats for inducing cardiac cell maturation or modeling pathological processes, we also employ 3D models in which cardiac myocytes and fibroblasts self-assemble into contracting cardiac microtissues (CMT) mounted on flexible beams. These beams provide biomechanical input and can report force generation in real time. We employ formats with tunable in vitro afterload to study load-dependent myocardial maturation, load-dependent dysfunction, the acceleration of cardiomyopathies by biomechanical stress and the molecular dynamics transducing these processes. The use of cardiac myocytes derived from human induced pluripotent stem cells (Hu-iPSC-CMs), molecular reporters and advances in gene editing provides diverse unprecedented opportunities to rigorously study these processes in human tissues. (Collaboration with Prosser and Turner laboratories)
Relevant Recent Publications
Vite A, Caporizzo MA, Corbin EA, Brandimarto J, McAfee Q, Livingston CE, Prosser BL, Margulies KB. Extracellular stiffness induces contractile dysfunction in adult cardiomyocytes via cell-autonomous and microtubule- dependent mechanisms. Basic Res Cardiol. 2022 Aug 25;117(1):41. PMID: 36006489.
Corbin EA, Vite A, Peyster EG, Bhoopalam M, Brandimarto J, Wang X, Bennett AI, Clark AT, Cheng X, Turner KT, Musunuru K, Margulies KB. Tunable and Reversible Substrate Stiffness Reveals a Dynamic Mechanosensitivity of Cardiomyocytes. ACS Appl Mater Interfaces. 2019; 11(23):20603-20614. PMID: 31074953
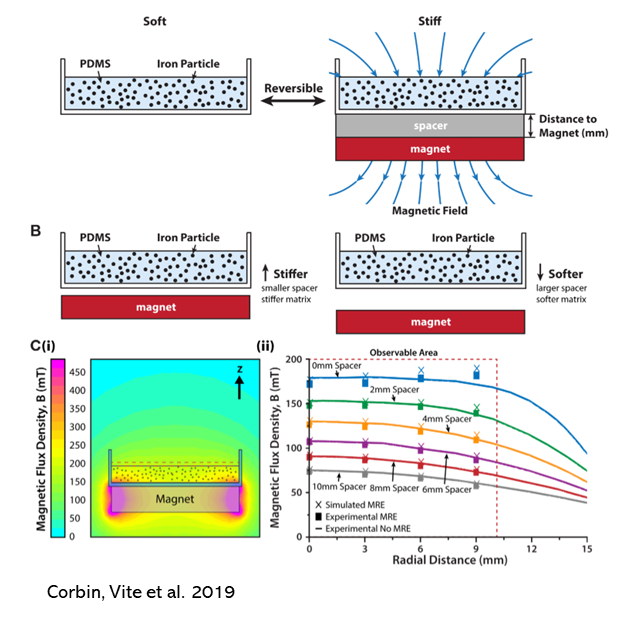
There is an unmet clinical need for agents that safely improve impaired contraction and relaxation observed in most patients with heart failure (HF). At present, all agents that augment contractility increase adverse outcomes and are considered palliative. Ongoing studies in the Prosser and Margulies labs demonstrate that the microtubule network (MTN) within each cardiac myocyte acts as a viscous restraint that impedes contraction and slows relaxation in HF. The stiffness and abundance of the MTN is regulated by the ratio of the detyrosinated and tyrosinated forms of α-tubulin (dT/T). More detyrosination, as we have observed in HF, is associated with greater stiffness, impairment of both contraction and relaxation and greater improvement in contractility following manipulations that decrease dT/T. Acute treatment with a small molecule vasohibin inhibitor improved myocardial relaxation both ex vivo (isolated rat and human cardiomyocytes) and in vivo using a rodent model of heart failure with preserved ejection fraction (HFpEF). Accordingly, our labs are actively addressing timely and strategic questions that are relevant to clinical translation of our recent findings including: 1) what causes increased MTN density in HF?; 2) does sustained targeting of the enzymes regulating dT/T improve cardiac contractility and relaxation without detrimental effects?; 3) Do interventions that decrease dT/T improve contractility without increasing myocardial oxygen consumption and/or ATP utilization?; and 4) Are there detrimental non-cardiac effects of sustained decreases in dT/T that necessitate preferential myocardial targeting via novel gene delivery methods. These inquiries involve utilization of super-resolution live-cell microscopy, engineered myocardial microtissues derived from human induced pluripotent stem cells, and animal models. (Collaboration with Prosser, Muzykantov and Weissman laboratories)
Relevant Recent Publications
Eaton DM, Lee BW, Caporizzo MA, Iyengar A, Chen CY, Uchida K, Marcellin G, Lannay Y, Vite A, Bedi KC, Jr., Brady CF, Smolyak JN, Meldrum D, Dominic J, Weingarten N, Patel M, Belec A, Hached K, Atluri P, Van Der Laan S, Prosser BL, Margulies KB. Vasohibin inhibition improves myocardial relaxation in a rat model of heart failure with preserved ejection fraction. Sci Transl Med. 2024. PMID: 39018366
Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, Morley MP, Margulies KB, Prosser BL. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med. 2018 Jun 11. [Epub ahead of print] PMID: 29892068
Caporizzo MA, Chen CY, Bedi K, Margulies KB, Prosser BL. Microtubules Increase Diastolic Stiffness in Failing Human Cardiomyocytes and Myocardium. Circulation. 2020 Jan 16. [Epub ahead of print] PMID: 31941365
The normal human heart consumes 6 kg of ATP daily with little capacity for substrate storage and possesses great flexibility to use alternative fuel substrates. Our recent studies have demonstrated, for the first time, the impact of reduced metabolic flexibility on the contractile function of human cardiomyocytes. With contractility as the primary readout, we find that failing hearts develop impairments in glucose utilization and become more reliant on a physiologic mix of glucose, fatty acids and ketones. We are employing isolated perfused human heart tissue preparations to better characterize shifts in substrate utilization associated with cardiomyopathies and therapeutic interventions, including SGLT2 inhibitors. In severely failing human hearts, we observe up-regulation in Ketone metabolism. Complementary findings in Dan Kelly’s lab using murine models demonstrate that increased burning of ketones in advanced heart failure is adaptive. These studies are currently being extended with clinical trials examining whether short-term ketone supplementation improves exercise performance in patients with heart failure. Our collaborator, Alexia Vite, is examining the mechanisms through which biomechanical overload promotes myocardial insulin resistance in order to inform strategies which may prevent or reverse maladaptive metabolic remodeling. (Collaboration with Kelly, Arany, and Vite laboratories)
Relevant Recent Publications
Vite A, Matsura TR, Bedi KC, Flam EL, Arany Z, Kelly DP, Margulies KB. Functional Impact of Alternative Metabolic Substrates in Failing Human Cardiomyocytes. JACC Basic Trans Sci 2023, in press
Flam E, Jang C, Murashige D, Yang Y, Morley MP, Jung S, Kantner DS, Pepper H, Bedi KC Jr, Brandimarto J, Prosser BL, Cappola T, Snyder NW, Rabinowitz JD, Margulies KB, Arany Z. Integrated landscape of cardiac metabolism in end-stage human nonischemic dilated cardiomyopathy. Nat Cardiovasc Res. 2022 Sep;1(9):817-829. Epub 2022 Aug 29. PMID: 36776621; PMCID: PMC9910091.
Selvaraj S, Fu Z, Jones P, Kwee LC, Windsor SL, Ilkayeva O, Newgard CB, Margulies KB, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Lanfear DE, Nassif ME, Javaheri A, Mentz RJ, Kosiborod MN, Shah SH; DEFINE-HF Investigators. Metabolomic Profiling of the Effects of Dapagliflozin in Heart Failure With Reduced Ejection Fraction: DEFINE-HF. Circulation. 2022 Sep 13;146(11):808-818. PMID: 35603596; PMCID: PMC9474658.
Inherited cardiomyopathies are a major cause of heart disease in all age groups and affect both patients and their families. Our lab is exceptionally well-positioned to model these diseases, identify the mechanisms through which “disease-enabling” mutations contribute to inherited cardiomyopathies and develop strategies to mitigate the pathological processes involved. To characterize the impact of pathogenic genetic variants, we employ models of inherited cardiomyopathies based on cardiac myocytes and engineered heart tissues derived from patient-specific induced pluripotent stem cell (Hu-iPSC-CMs). Our recent studies demonstrate that models which allow dynamic control of biomechanical inputs are particularly valuable for unmasking how genetic variants predispose to cardiomyopathies. Our capacity to compare findings in these models with characterizations of primary cardiac myocytes and myocardial slices from the individuals whose diseases we are modeling provides critical benchmarks for model validation and refinement. (Collaboration with Owens, Musunuru, Arany, Jain and Prosser laboratories)
Relevant Recent Publications
Lee BW, Caporizzo MA, Chen CY, Bedi KC, Peyster EG, Prosser BL, Margulies KB, Vite A. Adult human cardiomyocyte mechanics in osteogenesis imperfecta. Am J Physiol Heart Circ Physiol. 2023 Oct 1. doi: 10.1152/ajpheart.00391.2023. PMID: 37566108.
Ramachandran A, Livingston CE, Vite A, Corbin EA, Bennett AI, Turner KT, Lee BW, Lam CK, Wu JC, Margulies KB. Biomechanical Impact of Pathogenic MYBPC3 Truncation Variant Revealed by Dynamically Tuning In Vitro Afterload. J Cardiovasc Transl Res. 2023 Mar 6. doi: 10.1007/s12265-022-10348-4. Epub ahead of print. PMID: 36877449.
Previs MJ, O'Leary TS, Morley MP, Palmer BM, LeWinter M, Yob JM, Pagani FD, Petucci C, Kim MS, Margulies KB, Arany Z, Kelly DP, Day SM. Defects in the Proteome and Metabolome in Human Hypertrophic Cardiomyopathy. Circ Heart Fail. 2022 Jun;15(6):e009521. doi: 10.1161/CIRCHEARTFAILURE.121.009521. Epub 2022 May 11. PMID: 35543134; PMCID: PMC9708114.
Chaffin M, Papangeli I, Simonson B, Akkad AD, Hill MC, Arduini A, Fleming SJ, Melanson M, Hayat S, Kost-Alimova M, Atwa O, Ye J, Bedi KC Jr, Nahrendorf M, Kaushik VK, Stegmann CM, Margulies KB, Tucker NR, Ellinor PT. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature. 2022 Aug;608(7921):174-180. doi: 10.1038/s41586-022-04817-8. Epub 2022 Jun 22. PMID: 35732739.
In recent years, advanced machine learning techniques have been applied to a widening array of complex biological processes to gain new insights into underlying patterns that previously escaped detection. In this context, we have been applying machine learning algorithms to advanced analysis of images of myocardial histology to identify features of previously unappreciated diagnostic and prognostic value. We have demonstrated that digital pathology algorithms can distinguish failing vs. non-failing hearts with a sensitivity that far exceeds the performance of expert pathologists. Likewise, computerized-assisted grading of cardiac allograft rejection outperforms trained pathologists based on both consistency and accuracy. In cardiac allografts, our studies employing immunophenotyping of inflammatory cell infiltrates identifies an underappreciated role of anti-inflammatory immune-checkpoint molecules in limiting the severity of rejection. In addition, machine learning analysis of microvascular morphology at one year post-transplant can predict clinically significant allograft arteriopathy at 4+ years later. Ongoing studies using these and related techniques are integrating computer-assisted morphologic analyses with spatially-referenced molecular surveys. We are applying these techniques to identify mechanisms in inflammatory cardiomyopathies, heart failure with preserved ejection fraction and assessments of donor heart suitability for transplantation. (Collaboration with Peyster laboratory)
Relevant Recent Publications
Arabyarmohammadi S, Yuan C, Viswanathan VS, Lal P, Feldman MD, Fu P, Margulies KB, Madabhushi A, Peyster EG. Failing to Make the Grade: Conventional Cardiac Allograft Rejection Grading Criteria Are Inadequate for Predicting Rejection Severity. Circ Heart Fail. 2024 Feb 17. doi: 10.1161/CIRCHEARTFAILURE.123.010950. PMID: 38348670.
Peyster EG, Arabyarmohammadi S, Janowczyk A, Azarianpour-Esfahani S, Sekulic M, Cassol C, Blower L, Parwani A, Lal P, Feldman MD, Margulies KB, Madabhushi A. An automated computational image analysis pipeline for histological grading of cardiac allograft rejection. Eur Heart J. 2021 Jun 21;42(24):2356-2369. PMID: 33982079.
Peyster EG, Wang C, Ishola F, Remeniuk B, Hoyt C, Feldman MD, Margulies KB. In-situ Immune Profiling of Heart Transplant Biopsies Improves Diagnostic Accuracy and Rejection Risk Stratification. JACC Basic Transl Sci. 2020 Apr 1;5(4):328-340. PMID: 32368693
Peyster EG, Janowczyk A, Swamidoss A, Kethireddy S, Feldman MD, Margulies KB. Computational Analysis of Routine Biopsies Improves Diagnosis and Prediction of Cardiac Allograft Vasculopathy. Circulation. 2022 May 24;145(21):1563-1577. PMID: 35405081; PMCID: PMC9133227.

