Projects
Overview:
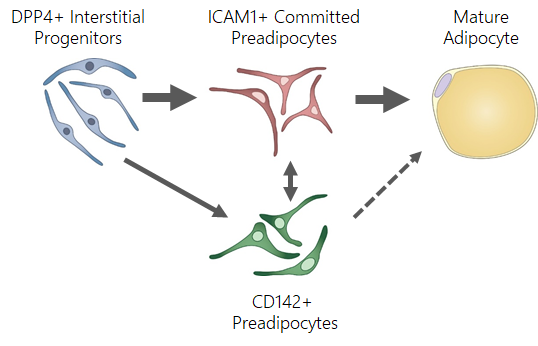
Adipose mesenchymal progenitors represent a complex pool of highly diverse cell types, for which we utilize unbiased techniques such as single-cell RNA transcriptomics to identify novel subpopulations of adipocyte progenitors from subcutaneous fat including Dpp4+ interstitial progenitor cells and Icam1+ committed preadipocytes. Dpp4+ progenitors demonstrate many properties classically attributed to mesenchymal stem cells including enhanced proliferative and multilineage differentiation potential. Icam1+ cells appear to be committed to the adipocyte lineage and are poised to undergo adipogenesis with minimal stimulation. Using cell transplantation and lineage tracing approaches, we have established a lineage hierarchy in which Dpp4+ cells serve as upstream progenitors that give rise to Icam1+ preadipocytes and mature adipocytes. Importantly, we found that the same cell populations and lineage relationships are present in human subcutaneous adipose. Adipocyte progenitor dysfunction, leading to inadequate de novo adipogenesis is thought to represent one of the main mechanisms contributing to the metabolic consequences of obesity. Therefore, understanding the physiological role of Dpp4+ and Icam1+ progenitor cells and how they are disrupted in the setting of obesity is of paramount importance. While the prevailing dogma in the field states that adipocyte progenitors are located in the perivascular space, we discovered that Dpp4+ progenitors reside in a previously unrecognized anatomic niche which we termed the Reticular Interstitium (RI), a previously overlooked connective tissue that envelops many organs. Furthermore, Dpp4+ progenitors cells demonstrated robust multilineage potential to differentiate into osteocytes and chondrocytes. The multipotent nature of Dpp4+ cells and their ubiquitous distribution throughout the body supports the hypothesis that, in addition to serving as precursors for adipocytes, these cells may play an important role in the development, regeneration and fibrosis of other mesenchymal tissues.
Current Projects:
In vivo lineage tracing of Dpp4+ interstitial progenitor cells
Adipose mesenchymal progenitors represent a complex pool of highly diverse cell types, for which we utilize unbiased techniques such as single-cell RNA transcriptomics to identify novel subpopulations of adipocyte progenitors from subcutaneous fat including Dpp4+ interstitial progenitor cells and Icam1+ committed preadipocytes. Dpp4+ progenitors demonstrate many properties classically attributed to mesenchymal stem cells including enhanced proliferative and multilineage differentiation potential. Icam1+ cells appear to be committed to the adipocyte lineage and are poised to undergo adipogenesis with minimal stimulation. Using cell transplantation and lineage tracing approaches, we have established a lineage hierarchy in which Dpp4+ cells serve as upstream progenitors that give rise to Icam1+ preadipocytes and mature adipocytes. Importantly, we found that the same cell populations and lineage relationships are present in human subcutaneous adipose. Adipocyte progenitor dysfunction, leading to inadequate de novo adipogenesis is thought to represent one of the main mechanisms contributing to the metabolic consequences of obesity. Therefore, understanding the physiological role of Dpp4+ and Icam1+ progenitor cells and how they are disrupted in the setting of obesity is of paramount importance.
Current projects are focused on testing the hypothesis that Dpp4+ progenitors give rise to Icam1+ committed preadipocytes and mature adipocytes in vivo during both embryologic adipose organogenesis and in the setting of stimulated adipogenesis in the adult; and that Dpp4+ progenitors may contribute to adipose fibrosis in the setting of high fat diet-induced obesity. To assess the role of endogenous Dpp4+ progenitor cells during adipogenesis in vivo, we have generated lineage tracing mice that leverage the specific expression of Dpp4 in the mesenchymal progenitor cells. We are using the Dpp4CreER model to track the fate of interstitial progenitor cells and their progeny as they progress through the stages of adipocyte or alternative fate commitment. Interstitial progenitor cells display many characteristics classically associated with traditional fibroblasts including the expression of collagen, fibronectin and matrix modulating genes. This gene pattern may reflect their likely role in maintaining the collagenous interstitium in which they normally reside, however these properties could also contribute to pathologic fibrosis if the Dpp4+ progenitors become activated in an ectopic location such as the parenchyma of the adipose tissue.
While the prevailing dogma in the field states that adipocyte progenitors are located in the perivascular space, we discovered that Dpp4+ progenitors reside in a previously unrecognized anatomic niche which we termed the Reticular Interstitium (RI), a previously overlooked connective tissue that envelops many organs. Furthermore, Dpp4+ progenitors cells demonstrated robust multilineage potential to differentiate into osteocytes and chondrocytes. The multipotent nature of Dpp4+ cells and their ubiquitous distribution throughout the body supports the hypothesis that, in addition to serving as precursors for adipocytes, these cells may play an important role in the development, regeneration and fibrosis of other mesenchymal tissues.
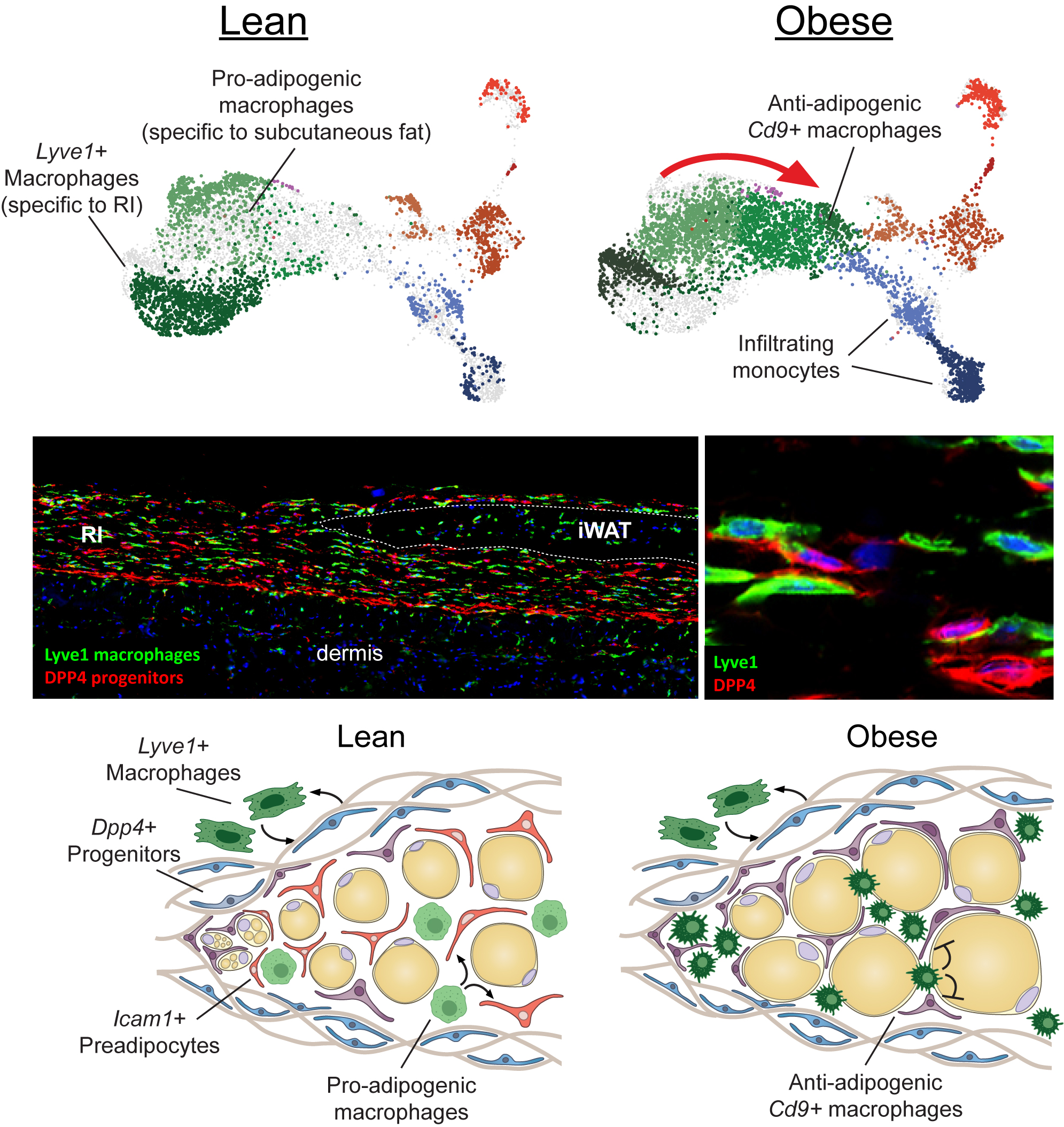
Characterization of reticular interstitium resident Lyve1+ macrophages
The reticular interstitium represents a previously under-recognized anatomical niche for progenitor cells and despite its potentially exciting role as a stem cell reservoir, the RI and its resident fibroblastic progenitors have only recently begun to receive intense scientific interest. We employed unbiased single cell RNA-sequencing to reveal that the RI is cohabited by Dpp4+ mesenchymal progenitors, as well as a unique population of macrophages marked by the expression of Lyve1. The close anatomic proximity of these two cell populations within the RI strongly suggests that Lyve1+ macrophages participate in immune-cell crosstalk with Dpp4+ cells and express niche defining elements that regulate Dpp4+ progenitor activity. Furthermore, we discovered a dramatic shift in the macrophage transcriptional profile in the setting of obesity: lean adipose contains pro-adipogenic macrophages; while obese adipose demonstrate a large influx of Cd9+ macrophages that are reportedly anti-adipogenic. These findings support the overarching hypothesis that Dpp4+ progenitors entering lean adipose encounter a pro-adipogenic macrophage signaling environment that is conducive to de novo adipogenesis, while Dpp4+ progenitors recruited into obese adipose tissue are met with anti-adipogenic/ pro-inflammatory Cd9+ macrophages, which might direct them into a fibrotic myofibroblast cell fate. We are actively investigating this hypothesis using FACS and single-cell to uncover candidate receptor-ligand gene set relationships existing between the Dpp4+ progenitors and Lyve1+ macrophages. We have developed an inducible in vivo macrophage depletion model to examine the functional consequences of Dpp4+ immune cell crosstalk as it relates to adipogenesis and fibrosis.