Research
Overview
Our lab aims to understand the cellular and molecular basis governing the formation, maintenance and function of neural circuits. We have established a platform combining animal models in flies and mammals, in vitro and in vivo, integrated with our bioengineering expertise. We focus on three main areas: elucidating the mechanisms underlying neural de- and regeneration; modeling human neurodevelopmental disorders in flies; and modeling brain tumorigenesis in flies. Our long-term goal is to provide the basis for designing novel therapies for treating neural injury and neurodevelopmental/neurodegenerative diseases.
Neural regeneration
Neural regeneration: Axons in the mature central nervous system (CNS) fail to regenerate after injury due to the loss of neuronal intrinsic growth potential and the extrinsic inhibitory environment, leading to sensory or motor dysfunctions, or paralysis in conditions such as spinal cord injury (SCI) or stroke. Even in the peripheral nervous system (PNS), where neurons are generally capable of axon regeneration, neurological deficits occur due to the slow rate of spontaneous axon regeneration, failure of reinnervation and the development of chronic pain. Few therapies for SCI or stroke exist due to our insufficient knowledge of the regeneration machinery and the lack of access to primate injury models to translate findings from lower organisms. Our lab has developed technologies to break these two barriers.
Boosting a neuron’s intrinsic growth potential is critical to regeneration therapy. We demonstrated that mechanical force, which is sensed by the mechanosensitive (MS) ion channel Piezo, inhibits axon regeneration. During regeneration, the growth cone physically interacts with the environment, generating a local force resulting in Piezo activation at the growth cone tip (Song et al., Neuron 2019). This in turn activates Atr (ataxia telangiectasia and Rad3 related) to cause regeneration failure (Li et al., Nat Comm 2021). Our work provided comprehensive mechanistic insights into how mechanical force controls nervous system maintenance. We were the first to implicate the MS ion channel Piezo (the identification of which was awarded the Nobel Prize in 2021) in regeneration and uncovered its downstream molecular pathway. In short, we identified a non-canonical function of the Atr pathway, which is conventionally known for its role in DNA damage response. In addition to this discovery, we also identified an RNA splicing/repair program that inhibits regeneration. Previously, we showed that Rtca (RNA 3’-terminal phosphate cyclase) restricts axon regeneration by delaying the nonconventional splicing of Xbp1 mRNA under cellular stress (Song et al., Nat Neurosci 2015). We have now identified the downstream effectors by transcriptome profiling, and showed that microtubule regulators, the secretory pathway and MS ion channels are involved. In the case of the former, we found that the microtubule associated protein, TPPP3, acts as a hub for microtubule regulators and relays cellular status information such as stress to dynamically instruct neuroregeneration via microtubules (Vargas et al., Genes Dev 2020). 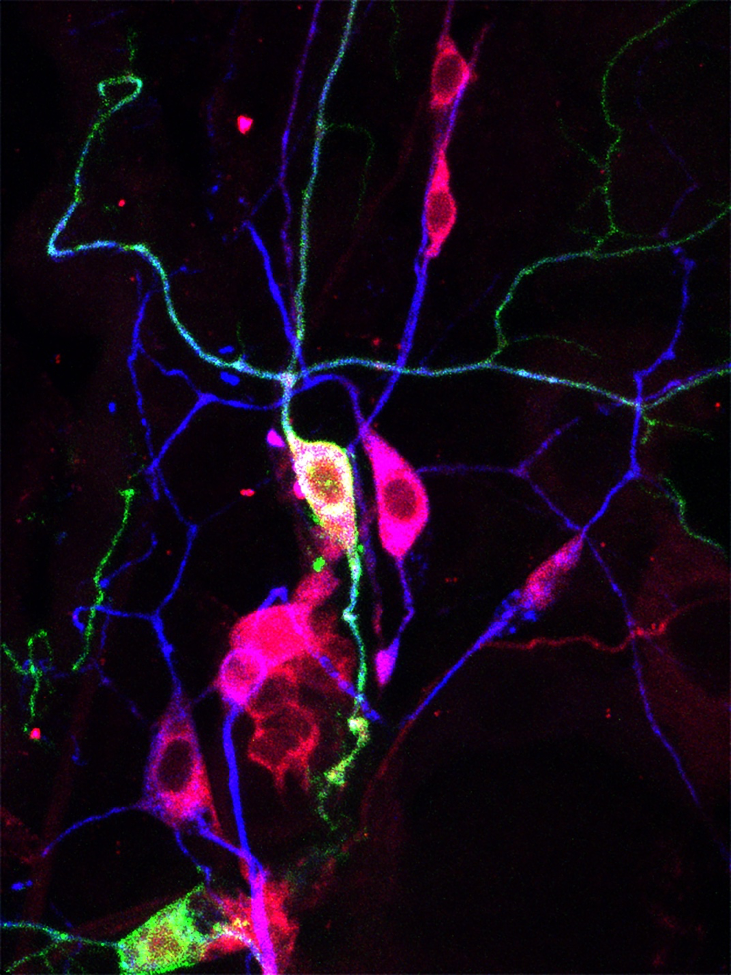
We also investigated the inhibitory microenvironment for CNS regeneration and found that it is reversible and depends on glial metabolic status. We showed that glia can be reprogrammed to promote not only morphological but also functional regeneration after CNS injury via increased glycolysis. This enhancement is mediated by the glia-derived metabolites lactate and L-2-hydroxyglutarate (L-2HG), which act on neuronal metabotropic GABAB receptors to boost cAMP signaling. Excitingly, the effect of metabolic reprogramming in promoting regeneration is evolutionarily conserved in mammals. Our findings not only provided invaluable insights into the inhibitory environment in the injured CNS, but also revealed a metabolic switch to circumvent the glial inhibition while amplifying their beneficial effects for treating CNS trauma (Li et al., Cell Metab 2020).
Currently, we are developing tools for stimulating or interrogating neuroregeneration. Using an optogenetic system, we can enhance regeneration and achieve temporal tuning and proper guidance of regenerating axons (Wang et al., eLife 2020), one of the remaining critical hurdles in the therapeutic regeneration field. Our latest endeavor is designing a CRISPR-based microfluidics platform for unbiased genetic screening of neural de- and regeneration.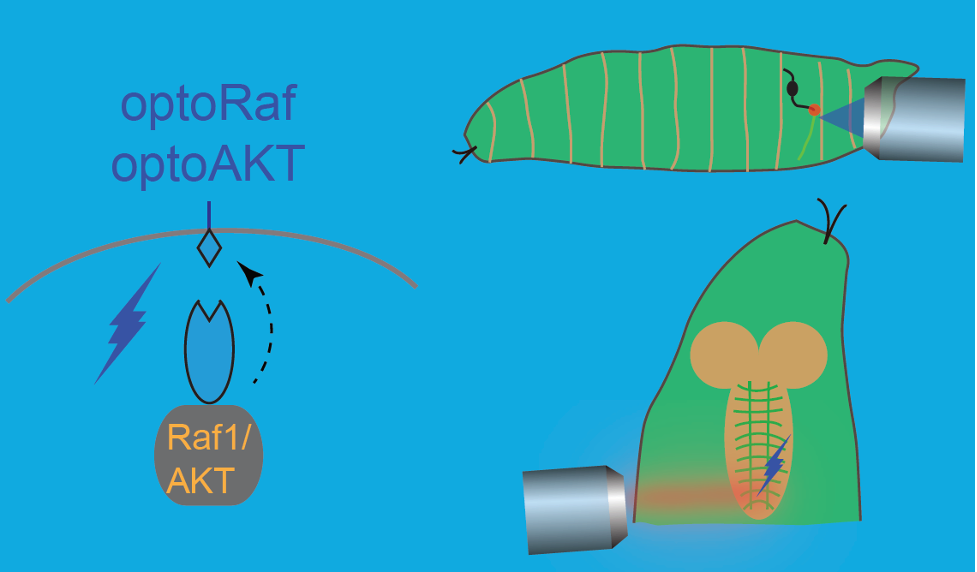
Neurodevelopmental diseases
Neurodevelopmental diseases: Intellectual disability encompasses a wide spectrum of neurodevelopmental disorders, with many linked genetic loci. However, the underlying molecular mechanism for more than 50% of patients remains elusive. In collaborative studies with CHOP colleagues, we are using fly models to determine the pathogenicity of genetic variants identified in human patients with neurodevelopmental disorders and intellectual disability, with the goal of better defining the underlying consequences of those mutations on brain dysfunction. Examples of this work include our identification of pathogenic variants in SMARCA5 encoding the ATPase motor of the ISWI chromatin remodeler as a cause of a previously unidentified neurodevelopmental syndrome. Our work showed the role of the ISWI family proteins in neural circuit formation and behavior (Li et al., Sci Adv 2021), which may in part explain the patients' phenotypes of developmental delay and microencephaly. We also found that bi-allelic loss-of-function of NUP188, an essential component of the nuclear- pore complex regulating nucleocytoplasmic transport of macromolecules, causes a recessive syndrome characterized by a distinct neurologic phenotype (Muir et al., AJHG 2020). Our work suggests that screening of NUP188 should be considered in infants with neurologic deficits. Our ongoing work has also identified pathogenic variants in pre-mRNA spliceosomal subunits causing syndromic autism. Our hope is to establish a platform intersecting clinical diagnosis, bioinformatic analyses, modeling in flies, induced pluripotent stem cells (iPSC) derived neurons and organoids, for systematically identifying and studying causal mutations underlying syndromic autism, and designing therapeutic strategies.
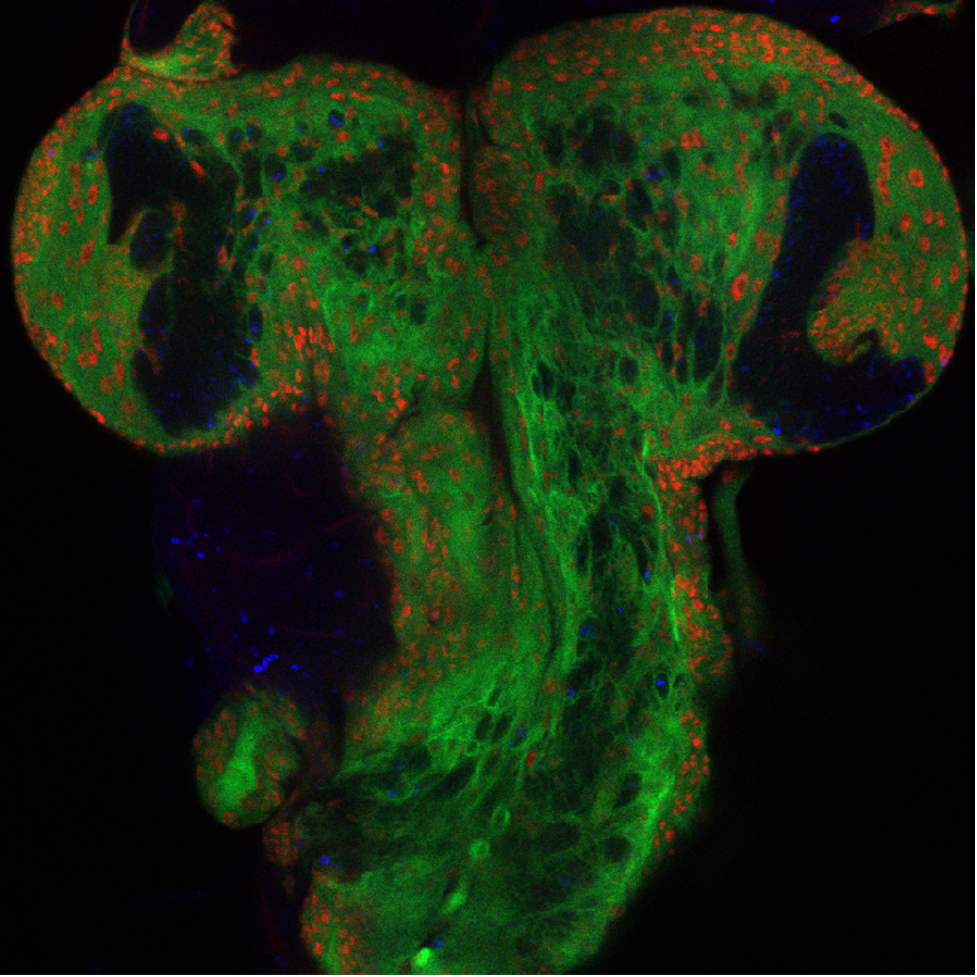 Tumorigenesis
Tumorigenesis
Tumorigenesis: We have been using flies to model pediatric low-grade gliomas (PLGGs) and glioblastoma. Pediatric brain tumors are one of the leading causes of disease related death in children, with PLGGs as the most commonly diagnosed sub-type. Despite its prevalence, the underlying pathogenesis is not well understood. In collaboration with CHOP colleagues, we are developing a customizable PLGG platform in flies, combined with bioinformatics from patient tumors.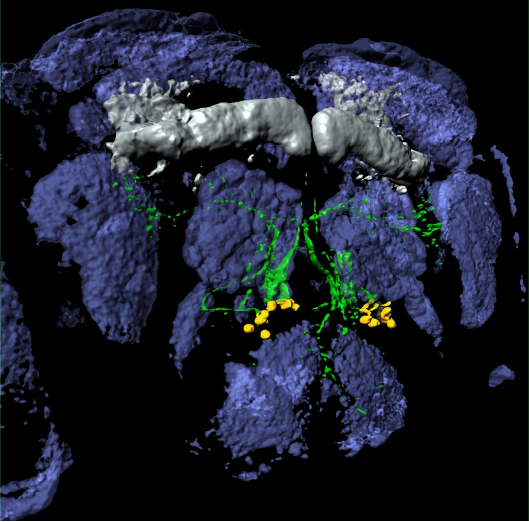
Potential Rotation Projects
- Utilize the fly sensory neuron injury model to identify novel pathways regulating degeneration and regeneration
- Study tumorigenesis in flies
- Establish new injury models using human cells
- CRISPR screen for neural regeneration and degeneration
AND MORE!

