Selected research publications
Selected Research Publications ( Complete list can be found at http://www.ncbi.nlm.nih.gov/pubmed?otool=upennlib&term=Amaravadi+RK)
Commentaries
- Amaravadi, Ravi K.: Autophagy-induced tumor dormancy in ovarian cancer. Journal of Clinical Investigation 118(12): 3837-40, Dec 2008. PMCID: PMC2582935
- Amaravadi RK. Autophagy in tumor immunity, Science 334(6062): 1501-2 2011 Dec 16PMID:22174234 PMC n/a
- Amaravadi RK, Debnath J : Mouse models address key concerns about autophagy inhibition for cancer therapy Cancer Discovery 4(8): 873-5, August 2014
- Amaravadi RK. Transcriptional regulation of autophagy in Ras-driven cancers. J. Clin Investigation. 2015 Apr 1;125(4):1393-5. doi: 10.1172/JCI81504. Epub 2015 Mar 23.PMID:25798614
Close
Autophagy is a resistance mechanism to chemotherapy in vivo
Amaravadi, Ravi K. Yu, Duonan. Lum, Julian J. Bui, Thi. Christophorou, Maria A. Evan, Gerard I. Thomas-Tikhonenko, Andrei. Thompson, Craig B.: Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. Journal of Clinical Investigation 117(2): 326-36, Feb 2007.
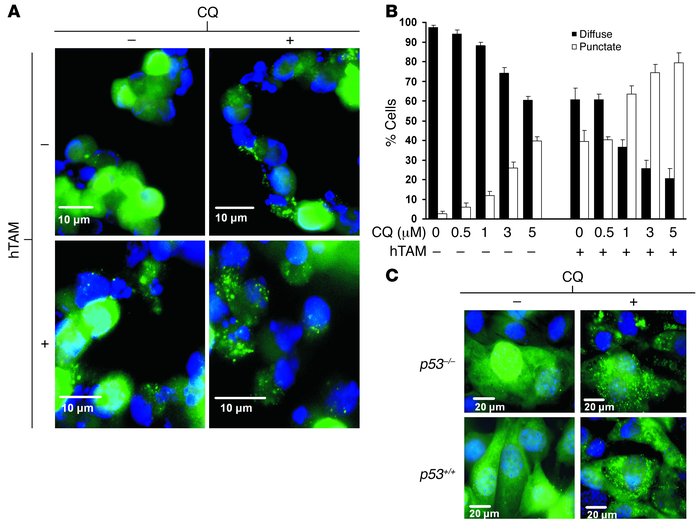 Significance: This was one of the first papers to demonstrate autophagy promotes resistance to chemotherapy in vivo, and targeting autophagy with chloroquine enhances the efficacy of chemotherapy.
Significance: This was one of the first papers to demonstrate autophagy promotes resistance to chemotherapy in vivo, and targeting autophagy with chloroquine enhances the efficacy of chemotherapy.
Close
Autophagy levels promote agressiveness and resistance to therapy in melanoma
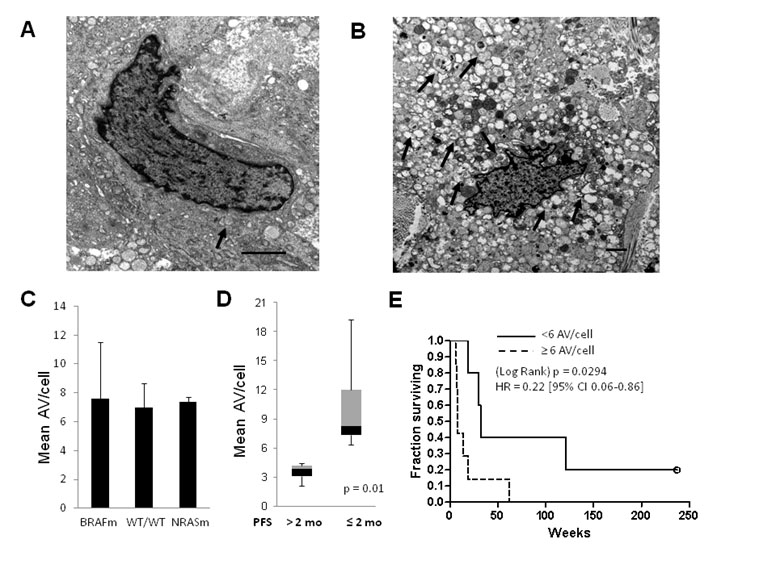
Ma Xiao-Hong, Piao Shengfu, Wang Dan, McAfee Quentin W, Nathanson Katherine L, Lum Julian J, Li Lin Z, Amaravadi Ravi K: Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clinical Cancer Research 17(10): 3478-89, May 2011. PMCID: PMC3096713 http://www.ncbi.nlm.nih.gov/pubmed/21325076
Significance: This was the first paper to show autophagy is a resistance mechanism in melanoma
Close
Lys05, a dimeric chloroquine is a more potent autophagy inhibitor than HCQ
McAfee QM, Zhang Z, SamantaA, Levi S, MaXH, Piao S, Lynch J,Sepulveda AR, DavisLE, Winkler J,Amaravadi RK: The autophagy inhibitor Lys05 has single agent antitumor activity reproduces the intestinal phenotype of a genetic autophagy deficiency. Proceedings of the National Academy of Sciences 109(21): 8253-8, May2012. PMCID: 22566612 http://www.ncbi.nlm.nih.gov/pubmed/22566612
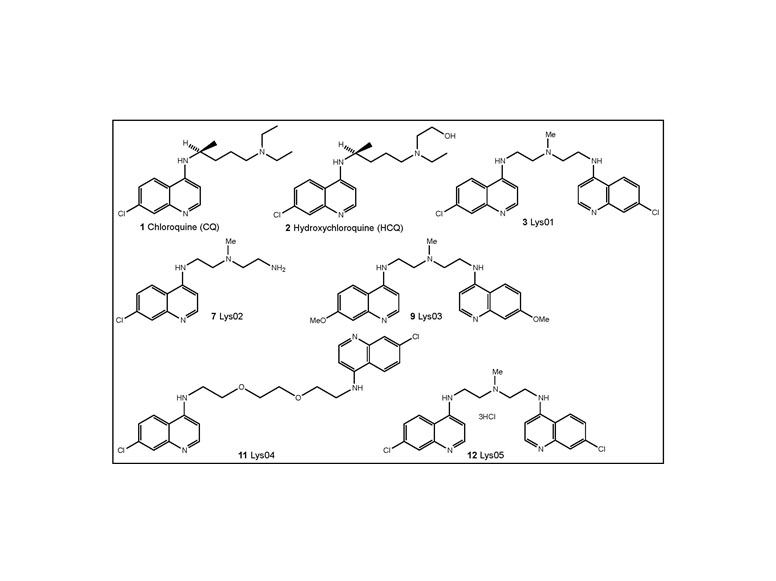
Significance:
First report of dimeric chloroquine derivative that is more potent than monomeric chloroquine derivatives at autophagy inhibition. This agent had single agent antitumor activity in multiple xenografts models.
Close
Er stress-associated autophagy is a resistance mechanism to BRAF inhibitors in melanoma
Ma XH, Piao SF, Dey S, Mcafee Q,Karakousis G, Villaneuva J , HartLS, Levi S, Hu J, Lazova R, Klump V, Pawelek JM,Xu X, Xu W,Schuchter LM,Davies MA, Herlyn M, Winkler J,KoumenisC,Amaravadi RK.: Targeting ER stress induced-autophagy overcomes resistance to BRAF inhibition in melanomaJournal of Clinical Investigation .124(3):1406-17PMID:24569374 PMCID:PMC3934165 http://www.ncbi.nlm.nih.gov/pubmed/24569374
 Significance:
Significance:
Autophagy levels were increased in tumors that had grown resistant to BRAF inhibtors in patients. In sensitive and resistant cell lines, BRAF inhibitors, and the combination of BRAF and MEK inhibition induce cytoprotective autophagy through the engagment of the ER stress response. Drug dependent engagement of mutant BRAF and the ER stress gatekeeper GRP78 was the mechanism by which ER stress is activated. Inhibition of autophagy enhanced the efficacy of BRAF inhibition in a BRAF inhibitor resistant mouse model of melanoma.
Close
Autophagy-dependent secreted proteins provide a snapshot of intratumoral autophagy dynamics
Identification of secreted proteins that reflect autophagy dynamics within tumor cells.Kraya AA1, Piao S, Xu X, Zhang G, Herlyn M, Gimotty P, Levine B, Amaravadi RK, Speicher DW.Autophagy 2015;11(1):60-74 PMID:25484078 PMCID:PMC4502670 http://www.ncbi.nlm.nih.gov/pubmed/25484078
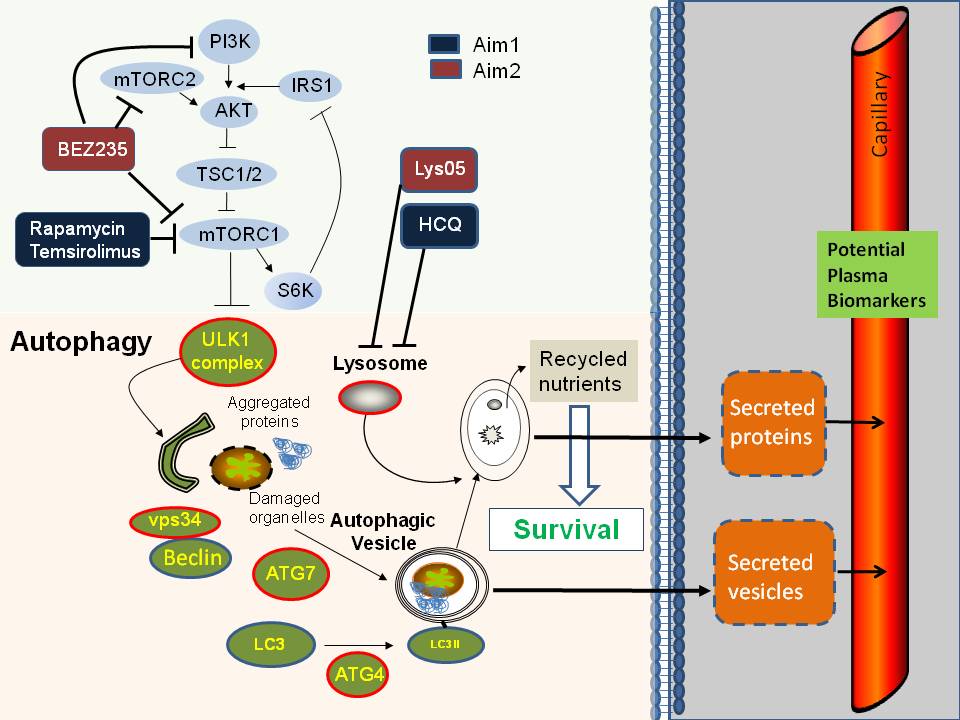 Significance: Comparison of the secretomes by unsupervised label free proteomics from three dimensional spheroids generated from a pair of isogenic melanoma cell lines with either high versus low autophagy levels identified a subset of inflammatory cytokines that were secreted in an autophagy dependent manner. These candidate biomarkers of intratumoral autophagy dynamics were elevated in the serum of patients with high levels of autophagy in their tumors.
Significance: Comparison of the secretomes by unsupervised label free proteomics from three dimensional spheroids generated from a pair of isogenic melanoma cell lines with either high versus low autophagy levels identified a subset of inflammatory cytokines that were secreted in an autophagy dependent manner. These candidate biomarkers of intratumoral autophagy dynamics were elevated in the serum of patients with high levels of autophagy in their tumors.
Close
First Clinical Trials of HCQ in cancer patients
- Rosenfeld MR, Supko JG, Grossman SA, Brem S, Mikkelson T, Wang D, Chang C, Hu J, McAfee Q, Troxel A, Piao S, Hetjian D, Tan KS, Pontiggia L, O’Dwyer PJ, Davis LE, Amaravadi RK. A Phase I/II Trial of Hydroxychloroquine in Conjunction with Radiation Therapy and Concurrent and Adjuvant Temozolomide in Patients with Newly Diagnosed Glioblastoma Multiforme. Autophagy 2014; Aug;10(8):1359-68 PMID: 24991840 PMCID: PMC4203513
- Rangwala R, Chang C, Hu J, Algazy K, Evans T, Fecher L, Schuchter LM, Torigian D, Troxel A, Tan KS, Hetjian DF, Demichele A, Vaughn D, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O'Dwyer PJ, Amaravadi RK. Combined mTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma Autophagy 2014; 10 (8). Aug;10(8):1391-402 PMID: 24991838 PMCID: PMC4203516
- Rangwala R, Leone R, Chang YC, Fecher L, Schuchter L, Kramer A, Tan KS, Heitjan DF, Rodgers G, Gallagher M, Piao S, Troxel A, Evans T, Demichele A, Nathanson KL, O'Dwyer PJ, Kaiser J, Pontiggia L, Davis LE, Amaravadi RK.: Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma Autophagy 10(8), August 2014.PMID: 24991839 PMCID: PMC4203514
- Vogl DT, Stadtmauer EA, Heitjan DF, Tan KS, Rangwala R, Piao S, Chang C, Scott EC, Paul TM, Nichols CW, Porter DL, Kaplan J, Mallon G, Bradner JE, Davis LE, Pontiggia L, Amaravadi RK. Combined autophagy and proteasome inhibition: Phase I trial of hydroxychloroquine with bortezomib in patients with relapsed refractory multiple myeloma. Autophagy 2014; Aug;10(8):1380-90 PMID: 24991834 PMCID: MC4203515
The PDFs can be found here:
http://www.ncbi.nlm.nih.gov/pubmed/24991834
http://www.ncbi.nlm.nih.gov/pubmed/24991840
http://www.ncbi.nlm.nih.gov/pubmed/24991838
http://www.ncbi.nlm.nih.gov/pubmed/24991836
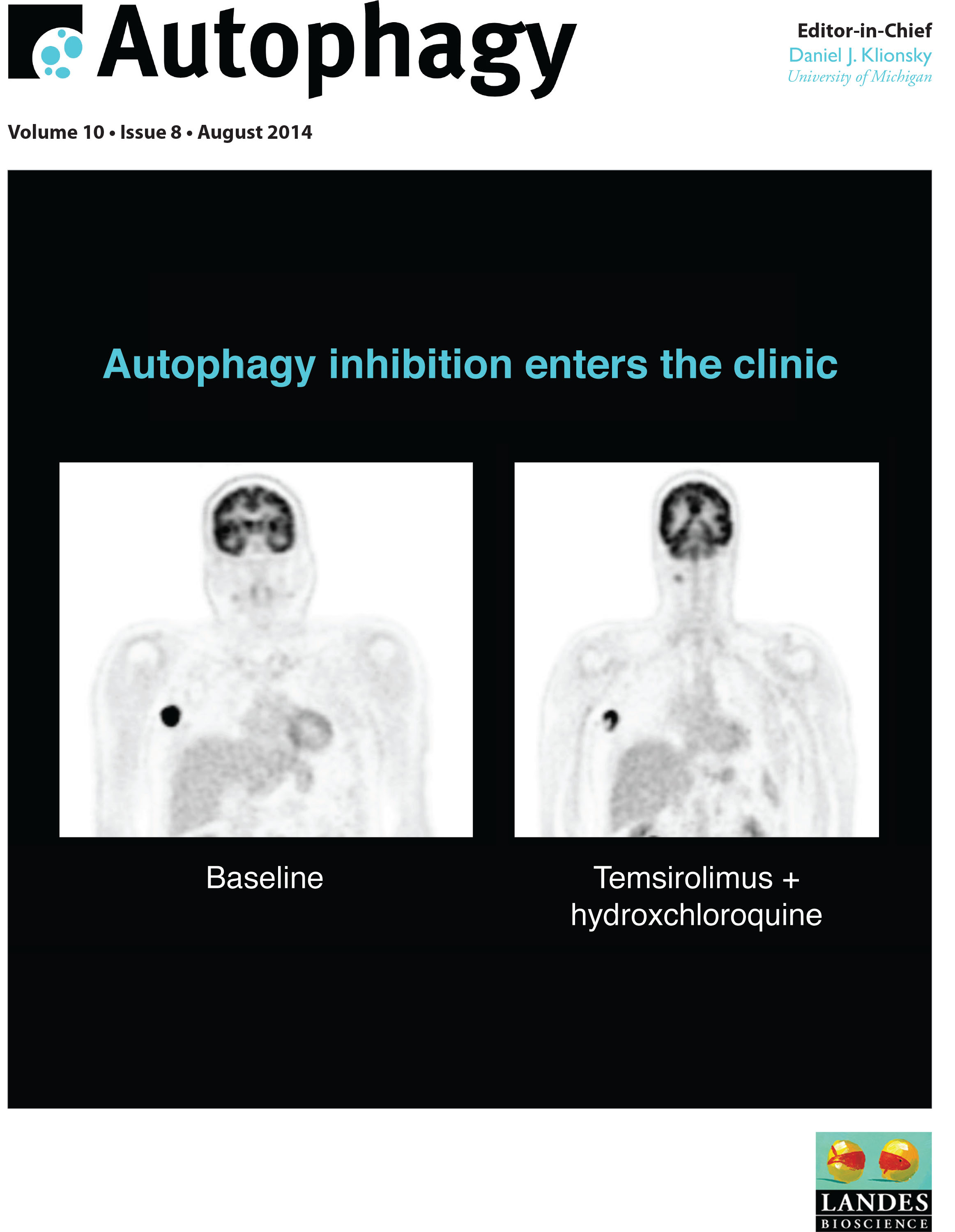 Significance: Six phase I/II trials were performed in human patients diagnosed with glioblastoma multiforme, relapsed/refractory myeloma , and melanoma in addition to other advanced tumors. One additional clinical trial was published wherein pet dogs diagnosed with spontaneously occurring lymphoma were also treated with HCQ-based combination therapies. Each trial involved a combination therapy that had preclinical studies to justify clinical translation. The major finding from these trials is that, based on electron microscopy-based pharmacodynamic assays, autophagy can be modulated therapeutically with chloroquine derivatives. Remarkably, across all of the trials <10% of patients had severe non-hematological toxicity. Specifically, there was no evidence of extensive metabolic toxicity, liver injury or neurologic impairment in these trials despite some evidence that chronic modulation of autophagy was achieved in patients, as seen by accumulation of autophagic vesicles in peripheral blood mononuclear cells and tumor cells. When combined with radiation therapy and concurrent and adjuvant temozolomide, HCQ produced dose-limiting myelosuppression at doses above 600 mg HCQ. At these doses only a subset of patients had evidence of autophagy modulation detectable in their peripheral blood mononuclear cells (PBMC), which may be one reason there was no significant improvement in overall survival compared to historical controls of temozolomide and radiation alone. Significant therapy-associated increases in AVs and LC3-II were observed in PBMCs in a concentration-dependent manner, demonstrating HCQ could modulate autophagy in vivo. Combined treatment with the proteasome inhibitor bortezomib and HCQ resulted in a greater perturbation of tumor cell autophagy compared to PBMC autophagy, arguing HCQ may selectively accumulate in tumor cells. Similar results were observed in the phase I trial of vorinostat and HCQ and in the canine lymphoma trial using doxorubicin with HCQ. Although these phase I studies were not powered to determine efficacy, response rates in unselected patient populations were generally low. However, there were a number of striking responses and prolonged stable disease observed in patients with melanoma, renal cell carcinoma, colon cancer and myeloma, that suggest a specific subset of cancers may be susceptible to regimens containing chloroquine-based autophagy inhibitors.
Significance: Six phase I/II trials were performed in human patients diagnosed with glioblastoma multiforme, relapsed/refractory myeloma , and melanoma in addition to other advanced tumors. One additional clinical trial was published wherein pet dogs diagnosed with spontaneously occurring lymphoma were also treated with HCQ-based combination therapies. Each trial involved a combination therapy that had preclinical studies to justify clinical translation. The major finding from these trials is that, based on electron microscopy-based pharmacodynamic assays, autophagy can be modulated therapeutically with chloroquine derivatives. Remarkably, across all of the trials <10% of patients had severe non-hematological toxicity. Specifically, there was no evidence of extensive metabolic toxicity, liver injury or neurologic impairment in these trials despite some evidence that chronic modulation of autophagy was achieved in patients, as seen by accumulation of autophagic vesicles in peripheral blood mononuclear cells and tumor cells. When combined with radiation therapy and concurrent and adjuvant temozolomide, HCQ produced dose-limiting myelosuppression at doses above 600 mg HCQ. At these doses only a subset of patients had evidence of autophagy modulation detectable in their peripheral blood mononuclear cells (PBMC), which may be one reason there was no significant improvement in overall survival compared to historical controls of temozolomide and radiation alone. Significant therapy-associated increases in AVs and LC3-II were observed in PBMCs in a concentration-dependent manner, demonstrating HCQ could modulate autophagy in vivo. Combined treatment with the proteasome inhibitor bortezomib and HCQ resulted in a greater perturbation of tumor cell autophagy compared to PBMC autophagy, arguing HCQ may selectively accumulate in tumor cells. Similar results were observed in the phase I trial of vorinostat and HCQ and in the canine lymphoma trial using doxorubicin with HCQ. Although these phase I studies were not powered to determine efficacy, response rates in unselected patient populations were generally low. However, there were a number of striking responses and prolonged stable disease observed in patients with melanoma, renal cell carcinoma, colon cancer and myeloma, that suggest a specific subset of cancers may be susceptible to regimens containing chloroquine-based autophagy inhibitors.
Close
Close
Dimeric quinacrine DQ661 concurrently inhibits mTOR and autophagy
Rebecca VW, Nicastri MC, McLaughlin N, Fennelly C, McAfee Q, Ronghe A, Nofal M, Lim CY, Witze E, Chude CI, Zhang G, Alicea GM, Piao S, Murugan S, Ojha R, Levi SM, Wei Z, Barber-Rotenberg JS, Murphy ME, Mills GB1, Lu Y, Rabinowitz J, Marmorstein R, Liu Q, Liu S, Xu X, Herlyn M, Zoncu R, Brady DC, Speicher DW, Winkler JD, Amaravadi RK. A unified approach to targeting the lysosome's degradative and growth signaling roles. Cancer Discovery 2017 7(11):1266-1283 PMID:28899863 PMCID:PMC5833978
Significance: We report a screen of novel dimeric antimalarials that identifies dimeric quinacrines (DQ) as potent anticancer compounds, which concurrently inhibit mTOR and autophagy. Central nitrogen methylation of the DQ linker enhances lysosomal localization and potency. An in situ photoaffinity pulldown identified palmitoyl-protein thioesterase 1 (PPT1) as the molecular target of DQ661. PPT1 inhibition concurrently impairs mTOR and lysosomal catabolism through the rapid accumulation of palmitoylated proteins. DQ661 inhibits the in vivo tumor growth of melanoma, pancreatic cancer, and colorectal cancer mouse models and can be safely combined with chemotherapy. Thus, lysosome-directed PPT1 inhibitors represent a new approach to concurrently targeting mTORC1 and lysosomal catabolism in cancer.
Close
PPT1 promotes tumor growth and is the molecular target of CQ derivatives
Rebecca VW, Nicastri MC, Fennelly C, Chude CI, Barber-Rotenberg J, Ronghe A, McAfee QM, McLaughlin N, Zhang G, Ojha R, Piao S, Schuchter L, Xu W, Gimotty P, Xiao X, Herlyn M, Marmorstein R, Speicher DW, Winkler JD, Amaravadi RK. PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Discovery. 2019; 9 (2):220-229 PMID: 30442709
Significance: Here, we report a novel dimeric CQ (DC661) capable of deacidifying the lysosome and inhibiting autophagy significantly better than hydroxychloroquine (HCQ). Using an in situ photoaffinity pulldown strategy, we identified palmitoyl-protein thioesterase 1 (PPT1) as a molecular target shared across monomeric and dimeric CQ derivatives. HCQ and Lys05 also bound to and inhibited PPT1 activity, but only DC661 maintained activity in acidic media. Knockout of PPT1 in cancer cells using CRISPR/Cas9 editing abrogates autophagy modulation and cytotoxicity of CQ derivatives, and results in significant impairment of tumor growth similar to that observed with DC661. Elevated expression of PPT1 in tumors correlates with poor survival in patients in a variety of cancers. Thus, PPT1 represents a new target in cancer that can be inhibited with CQ derivatives. SIGNIFICANCE: This study identifies PPT1 as the previously unknown lysosomal molecular target of monomeric and dimeric CQ derivatives. Genetic suppression of PPT1 impairs tumor growth, and PPT1 levels are elevated in cancer and associated with poor survival. These findings provide a strong rationale for targeting PPT1 in cancer.
Close
ER translocation drives resistance to targeted therapy by activating autophagy
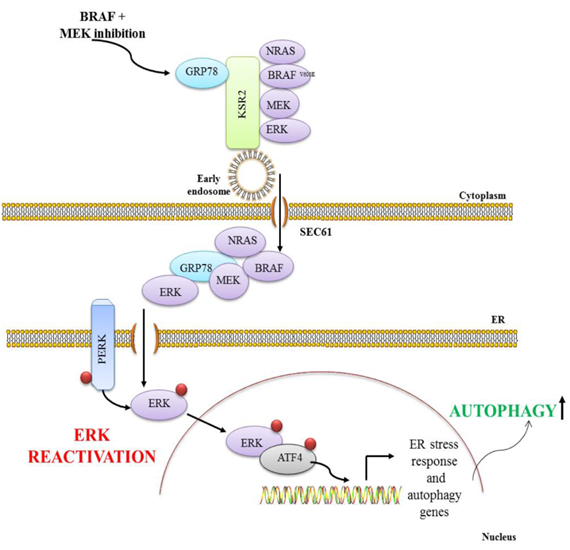
Ojha R, Leli NM, Onorati AM, Piao S, Verginadis II, Tameire F, Rebecca VW, Chude CI , Murugan S, Fennelly C, Noguera-Ortega E, Liu S, Xu X, Krepler C, Xiao M, Xu W, Gangadhar T, Karakousis G, Schuchter LM, Herlyn M, Koumenis C, Amaravadi RK. ER translocation of the MAPK pathway drives therapy resistance in BRAF mutant cancers. Cancer Discovery 2019; 9(3):396-415 PMID:30563872 PMCID:PMC6397701
Significance: ERK reactivation and autophagy are considered distinct resistance pathways to BRAF + MEK inhibition (BRAFi + MEKi) in BRAFV600E cancers. Here, we report BRAFi + MEKi–induced ER translocation of the MAPK pathway is necessary for ERK reactivation, which drives autophagy. The ER translocation mechanism is a major druggable driver of resistance to targeted therapy.
Close
PPT1 inhibition augments PD-1 checkpoint blockade in melanoma
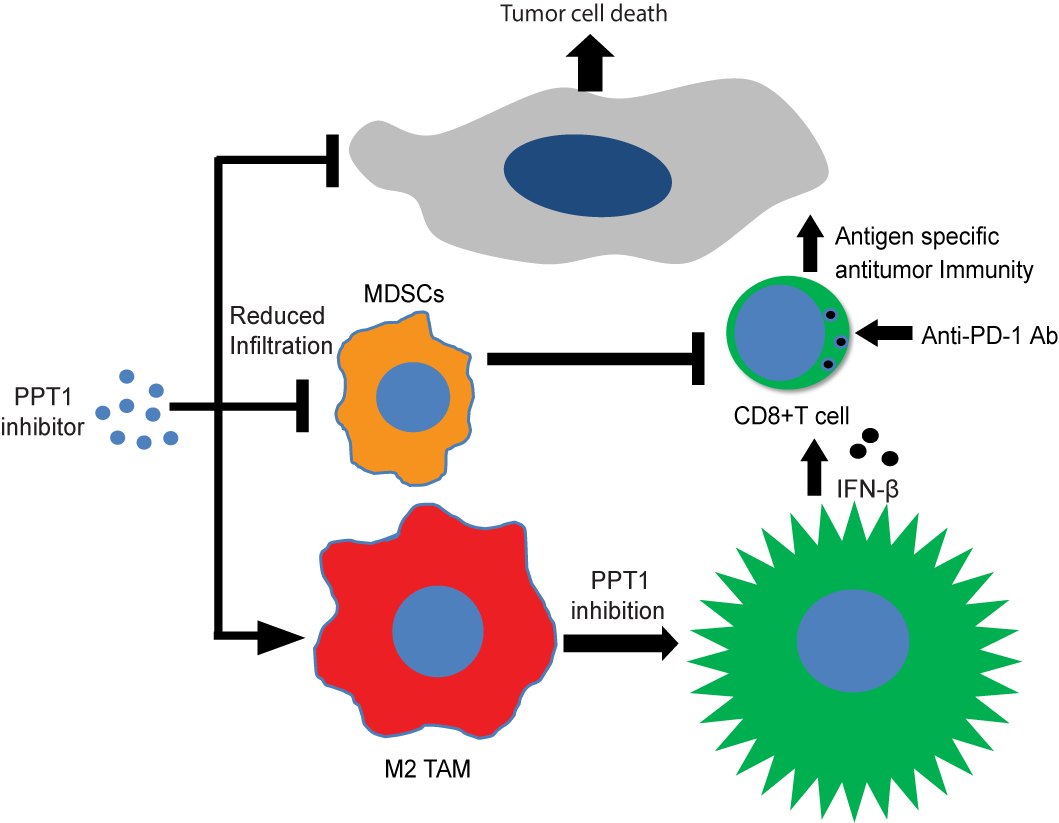
Sharma G, Ojha R, Noguera-Ortega E, Rebecca VW, Attanasio J, Liu S, Piao S, Lee J, Nicastri MC, Harper SL, Ronghe A, Jain V, Winkler JW, Speicher D, Mastio J, Gimotty PA, Xu X, Wherry EJ, Gabrilovich DI, Amaravadi RK. Ppt1 inhibition enhances the anti-tumor activity of anti-PD1 antibody in melanoma. JCI Insight 2020; 5(17):e133225. PMID: 32780726 PMCID: PMC7526447
Significance: New strategies are needed to enhance the efficacy of anti–programmed cell death protein antibody (anti–PD-1 Ab) in cancer. Here, we report that inhibiting palmitoyl-protein thioesterase 1 (PPT1), a target of chloroquine derivatives like hydroxychloroquine (HCQ), enhances the antitumor efficacy of anti–PD-1 Ab in melanoma. The combination resulted in tumor growth impairment and improved survival in mouse models. Genetic suppression of core autophagy genes, but not Ppt1, in cancer cells reduced priming and cytotoxic capacity of primed T cells. Exposure of antigen-primed T cells to macrophage-conditioned medium derived from macrophages treated with PPT1 inhibitors enhanced melanoma-specific killing. Genetic or chemical Ppt1 inhibition resulted in M2 to M1 phenotype switching in macrophages. The combination was associated with a reduction in myeloid-derived suppressor cells in the tumor. Ppt1 inhibition by HCQ, or DC661, induced cyclic GMP-AMP synthase/stimulator of interferon genes/TANK binding kinase 1 pathway activation and the secretion of interferon-β in macrophages, the latter being a key component for augmented T cell–mediated cytotoxicity. Genetic Ppt1 inhibition produced similar findings. These data provide the rationale for this combination in melanoma clinical trials and further investigation in other cancers.
Close
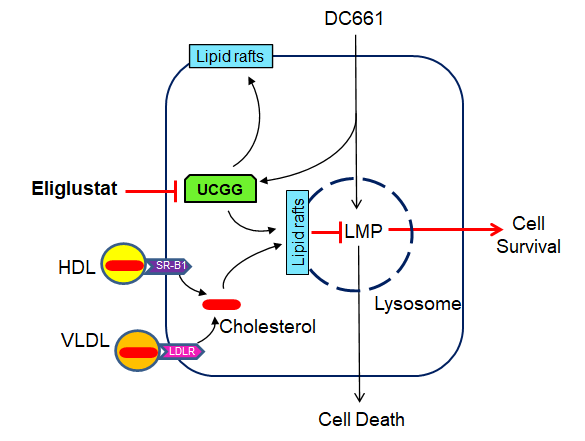
Targeting UGCG Overcomes Resistance to Lysosomal Autophagy Inhibition.
Jain V, Harper SL, Versace AM, Fingerman D, Brown GS, Bhardwaj M, Crissey MAS, Goldman AR, Ruthel G, Liu Q, Zivkovic A, Stark H, Herlyn M, Gimotty PA, Speicher DW, Amaravadi RK. Cancer Discov. 2023 Feb 6;13(2):454-473. doi: 10.1158/2159-8290.CD-22-0535. PMID: 36331284
Lysosomal autophagy inhibition (LAI) with hydroxychloroquine or DC661 can enhance cancer therapy, but tumor regrowth is common. To elucidate LAI resistance, proteomics and immunoblotting demonstrated that LAI induced lipid metabolism enzymes in multiple cancer cell lines. Lipidomics showed that LAI increased cholesterol, sphingolipids, and glycosphingolipids. These changes were associated with striking levels of GM1+ membrane microdomains (GMM) in plasma membranes and lysosomes. Inhibition of cholesterol/sphingolipid metabolism proteins enhanced LAI cytotoxicity. Targeting UDP-glucose ceramide glucosyltransferase (UGCG) synergistically augmented LAI cytotoxicity. Although UGCG inhibition decreased LAI-induced GMM and augmented cell death, UGCG overexpression led to LAI resistance. Melanoma patients with high UGCG expression had significantly shorter disease-specific survival. The FDA-approved UGCG inhibitor eliglustat combined with LAI significantly inhibited tumor growth and improved survival in syngeneic tumors and a therapy-resistant patient-derived xenograft. These findings nominate UGCG as a new cancer target, and clinical trials testing UGCG inhibition in combination with LAI are warranted.
Close
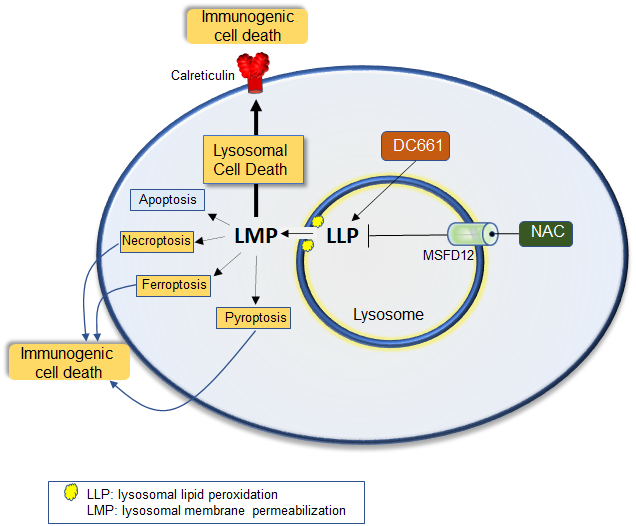
Lysosomal lipid peroxidation regulates tumor immunity.
Bhardwaj M, Lee JJ, Versace AM, Harper SL, Goldman AR, Crissey MAS, Jain V, Singh MP, Vernon M, Aplin AE, Lee S, Morita M, Winkler JD, Liu Q, Speicher DW, Amaravadi RK. J Clin Invest. 2023 Apr 17;133(8):e164596. doi: 10.1172/JCI164596. PMID: 36795483 PMCID: PMC10104903
Lysosomal inhibition elicited by palmitoyl-protein thioesterase 1 (PPT1) inhibitors such as DC661 can produce cell death, but the mechanism for this is not completely understood. Programmed cell death pathways (autophagy, apoptosis, necroptosis, ferroptosis, and pyroptosis) were not required to achieve the cytotoxic effect of DC661. Inhibition of cathepsins, or iron or calcium chelation, did not rescue DC661-induced cytotoxicity. PPT1 inhibition induced lysosomal lipid peroxidation (LLP), which led to lysosomal membrane permeabilization and cell death that could be reversed by the antioxidant N-acetylcysteine (NAC) but not by other lipid peroxidation antioxidants. The lysosomal cysteine transporter MFSD12 was required for intralysosomal transport of NAC and rescue of LLP. PPT1 inhibition produced cell-intrinsic immunogenicity with surface expression of calreticulin that could only be reversed with NAC. DC661-treated cells primed naive T cells and enhanced T cell-mediated toxicity. Mice vaccinated with DC661-treated cells engendered adaptive immunity and tumor rejection in "immune hot" tumors but not in "immune cold" tumors. These findings demonstrate that LLP drives lysosomal cell death, a unique immunogenic form of cell death, pointing the way to rational combinations of immunotherapy and lysosomal inhibition that can be tested in clinical trials.
Close
Reviews
- Amaravadi RK, Thompson CB.: The survival kinases Akt and Pim as potential therapeutic targets. Journal of Clinical Investigation 115(10): 2618, 2005
- Amaravadi RK, Thompson CB: The roles of therapy-induced autophagy and necrosis in cancer treatment. Clinical Cancer Research 13(24): 7271-9, Dec 15 2007
- Amaravadi RK, Lippincott-Schwartz J,Yin XM, Weiss WA, Takebe N,Timmer W, DiPaola RS, Lotze M, White E. Principles and Current Strategies for Targeting Autophagy for Cancer Treatment. Clinical Cancer Research 2011 Feb 15;17(4):654-666 PMID: 21325294 PMCID: PMC3075808
- Robert Leone, Ravi Amaravadi : Autophagy: a targetable linchpin of cancer cell metabolism. Trends in Endocrinology and Metabolism Cell Press,(4), 209-17, Apr 24 2013.
- Rebecca VW, Amaravadi RK : Emerging Strategies to effectively target autophagy in cancer Oncogene. 2016. PMID:25893285
- Amaravadi RK, Kimmelman A, White E. Recent Insights into the function of autophagy in cancer. Genes and Dev 2016 Sep 1;30(17):1913-30. PMID: 27664235 PMCID:PMC5066235
- Amaravadi RK, Kimmelman AC, Debnath J Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discovery. 2019 Sep. 9; (9):1167-1181. PMID:31434711
- Bhardwaj Monika, Leli Nektaria Maria, Koumenis Constantinos, Amaravadi Ravi K: Regulation of autophagy by canonical and non-canonical ER stress responses. Semin Cancer Biol. S1044-579X(19): 30394-3, Dec 12 2019.
-
Jain V, Singh MP, Amaravadi RK. Recent advances in targeting autophagy in cancer. Trends Pharmacol Sci. 2023 May;44(5):290-302. doi: 10.1016/j.tips.2023.02.003. Epub 2023 Mar 15. PMID: 36931971
Close
Divergent effects of acute and chronic PPT1 inhibition in melanoma
Crissey MAS, Versace A, Bhardwaj M, Jain V, Liu S, Singh A, Beer LA, Tang HY, Villanueva J, Gimotty PA, Xu X, & Amaravadi RK.
Autophagy 2025 Feb 24(2) :394-406. doi: 10.1080/15548627.2024.2403152. PMID: 39265628
 Macroautophagy/autophagy-lysosome function promotes growth and survival of cancer cells, making them attractive targets for cancer therapy. One intriguing lysosomal target is PPT1 (palmitoyl-protein thioesterase 1). PPT1 inhibitors derived from chloroquine block autophagy, have significant antitumor activity in preclinical models and are being developed for clinical trials. However, the role of PPT1 in tumorigenesis remains poorly understood. Here we report that in melanoma cells, acute siRNA or pharmacological PPT1 inhibition led to increased ferroptosis sensitivity and significant loss of viability, whereas chronic PPT1 knockout using CRISPR-Cas9 produced blunted ferroptosis that led to sustained viability and growth. Each mode of PPT1 inhibition produced lysosome-autophagy inhibition but distinct proteomic changes, demonstrating the complexity of cellular adaptation mechanisms. To determine whether total genetic loss of Ppt1 would affect tumorigenesis in vivo, we developed a Ppt1 conditional knockout mouse model. We then crossed it into the BrafCA, PtenloxP, Tyr:CreERT2 melanoma mouse model to investigate the impact of Ppt1 loss on tumorigenesis. Loss of Ppt1 had no impact on melanoma histology, time to tumor initiation, or survival of tumor-bearing mice. These results suggest that chemical PPT1 inhibitors produce different adaptations than genetic PPT1 inhibition, and additional studies are warranted to fully understand the mechanism of chloroquine derivatives that target PPT1 in cancer.
Macroautophagy/autophagy-lysosome function promotes growth and survival of cancer cells, making them attractive targets for cancer therapy. One intriguing lysosomal target is PPT1 (palmitoyl-protein thioesterase 1). PPT1 inhibitors derived from chloroquine block autophagy, have significant antitumor activity in preclinical models and are being developed for clinical trials. However, the role of PPT1 in tumorigenesis remains poorly understood. Here we report that in melanoma cells, acute siRNA or pharmacological PPT1 inhibition led to increased ferroptosis sensitivity and significant loss of viability, whereas chronic PPT1 knockout using CRISPR-Cas9 produced blunted ferroptosis that led to sustained viability and growth. Each mode of PPT1 inhibition produced lysosome-autophagy inhibition but distinct proteomic changes, demonstrating the complexity of cellular adaptation mechanisms. To determine whether total genetic loss of Ppt1 would affect tumorigenesis in vivo, we developed a Ppt1 conditional knockout mouse model. We then crossed it into the BrafCA, PtenloxP, Tyr:CreERT2 melanoma mouse model to investigate the impact of Ppt1 loss on tumorigenesis. Loss of Ppt1 had no impact on melanoma histology, time to tumor initiation, or survival of tumor-bearing mice. These results suggest that chemical PPT1 inhibitors produce different adaptations than genetic PPT1 inhibition, and additional studies are warranted to fully understand the mechanism of chloroquine derivatives that target PPT1 in cancer.
Close