Abnormal Mitochondrial Bioenergetic and Motility Signatures as Indices of Acute Poisoning in a Translational Approach from Bench to Bedside
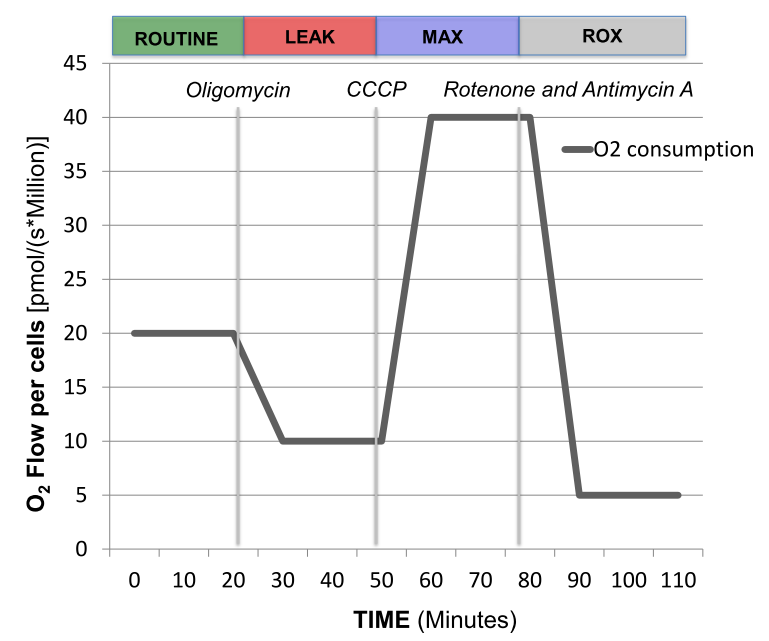
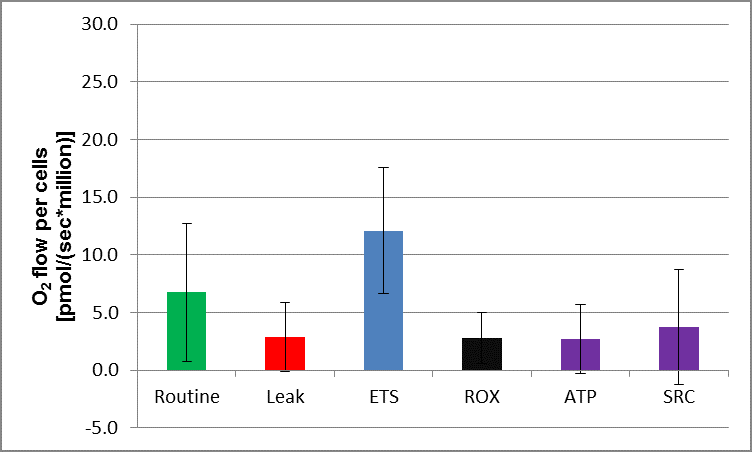
Representative tracing of oxygen consumption measured in intact peripheral blood mononuclear cells. The solid grey line shows the rate of oxygen consumption. After measuring ROUTINE oxygen consumption, the adenosine triphosphate (ATP)-synthase inhibitor oligomycin is added to obtain proton LEAK. The uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) allows the measurement of maximal oxygen consumption (MAX) stimulating maximal respiration assuming all required substrates are present. Finally, rotenone (Complex I inhibitor) and antimycin A (Complex III inhibitor) will completely prevent oxygen consumption through the electron respiratory chain. The remaining residual oxygen consumption (ROX) is attributed from other non-mitochondrial sources such as oxidases. ATP-linked oxygen consumption is calculated as routine respiration minus LEAK and spare respiratory capacity (SRC) is calculated as maximal respiration minus routine oxygen consumption.
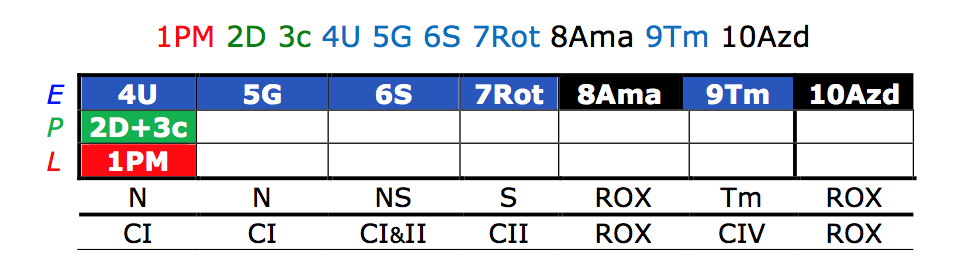
This is the sequence of injections given in platelets to obtain more comprehensive respiration measurements to assess for individual complex activity. The numerical sequence was the order in which the following injections were given with the corresponding complex activity found above: PM=Pyruvate and malate; D=ADP; c=cytochrome; U=CCCP; G=Glutamate; S=Succinate; Rot=Rotenone; Ama=Antimycin; Tm=Ascorbate and TMPD; Azd=Azide. Our protocol is an adaptation of Reference Protocol 1 developed by OROBOROS Instruments (Innsbruck, Austria).
The Eckmann Lab (Dr. Jang) is interested in studying the interaction of mitochondrial bioenergetics and dynamics in the area of acute care that includes sepsis and acute toxicologic poisoning. We are currently taking a translational approach studying the mitochondria at a cell-based level all the way to the clinical setting actively enrolling patients with these acute medical conditions with the goal to develop better prognostic measures with the potential for mitochondrial-directed therapy. The Eckmann lab has both the latest model of the OROBOROS O2K with Fluorometer and Seahorse XF24 to study mitochondrial respiration in both intact and permeabilized cells. In addition we also study mitochondrial dynamics examining both mitochondrial motility and fusion/fission events with microscopy. Combining these elements we aim to better understand the complex interactions of both bioenergetics and motility in issues of acute care to better improve patient care and outcomes.
The following represents the translational approach to the study of mitochondrial bioenergetics and dynamics in acute care:
1. The effect of cyanide on mitochondrial bioenergetics and dynamics in select cell line
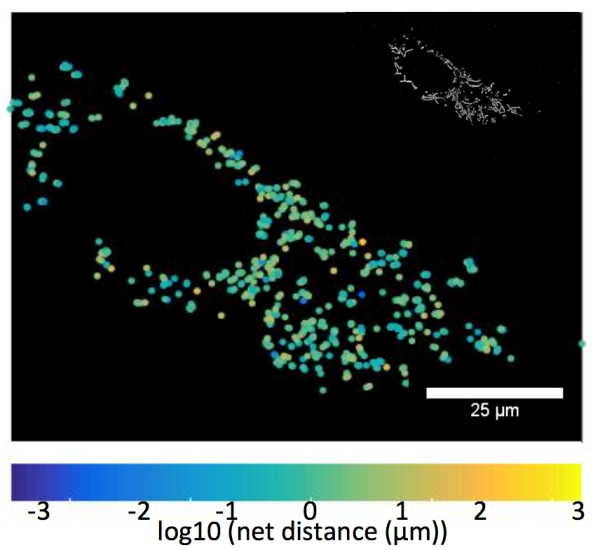
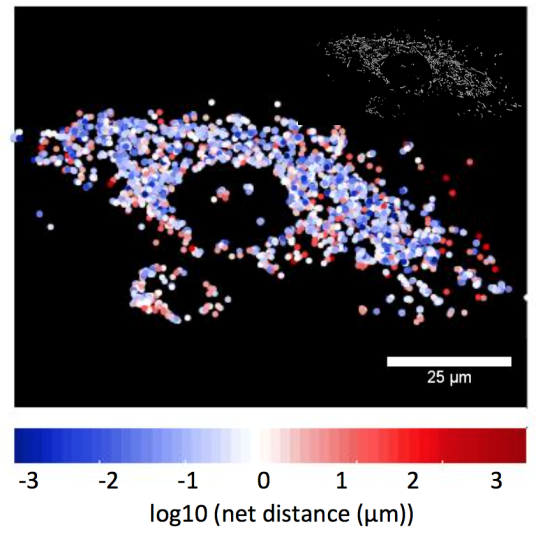
The left image demonstrates a HUVEC control cell showing the net distance traveled by labeled mitochondria. The right image demonstrates more perinuclear mitochondrial clustering and less mitochondrial movement in a HUVEC cell exposed to 100 mM cyanide for 30-minutes. Images are processed from the Eckmann lab to calculate mitochondrial net and total movement, and rates of fission/fusion.
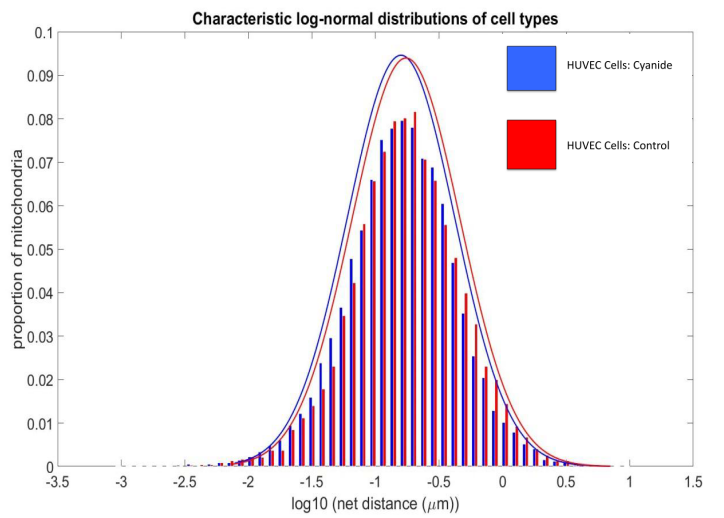
HUVEC control cells exhibit more mitochondria net distance movement when compared to HUVEC cells exposed to cyanide. Net distances appear to be log-normally distributed. R2=.9945
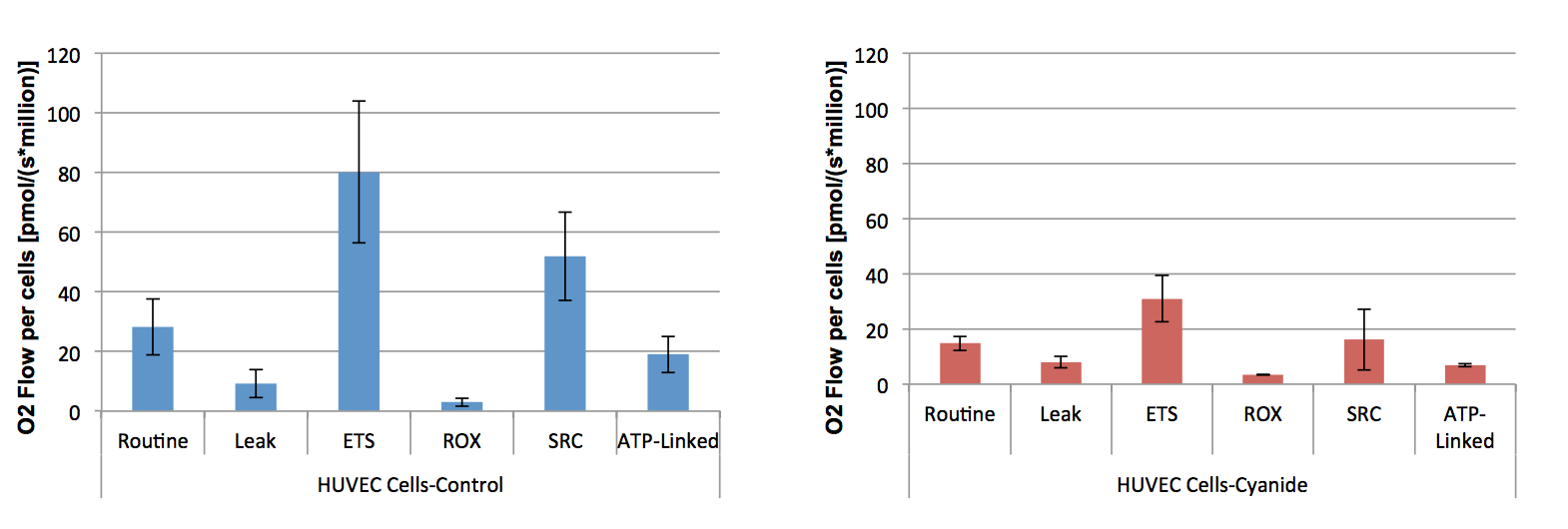
A series of OROBOROS experiments were performed for each condition (Control vs cyanide exposure) measuring respiration in intact HUVEC cells using a series of specific injections designed to evaluate key parameters in mitochondrial respiration. When compared to control cells, HUVEC cells exposed to cyanide for 30-minutes exhibited a significant decrease in routine respiration and maximal respiration (ETS) with no significant difference in leak and residual oxygen consumption (ROX). There was also a significant decrease in spare respiratory capacity (SRC) that is a measure of a cell’s fitness and ATP-linked respiration. The decrease in key parameters in mitochondrial respiration may explain the decrease in net mitochondrial net movement. *P=<0.05.
2. The measurement of mitochondrial respiration in human blood cells in carbon monoxide poisoning
Carbon monoxide (CO) is a colorless and odorless gas that is an important cause of poisoning mortality and morbidity in the United States. At this time there is no reliable method to predict severity of poisoning or clinical prognosis following CO exposure. Whole blood cells such as peripheral blood mononuclear cells (PBMCs) and platelets have been explored for their potential to act as sensitive biomarkers for mitochondrial dysfunction.
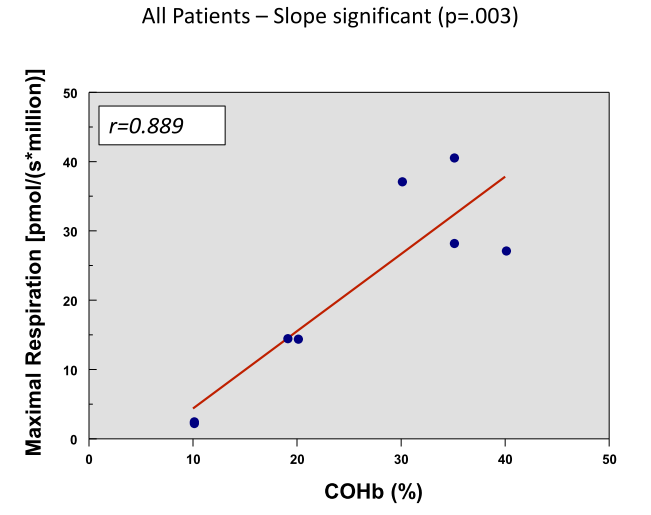
This graph shows a positive correlation of maximal respiration with the COHb level which may be a better reflection of clinical status than the COHb level
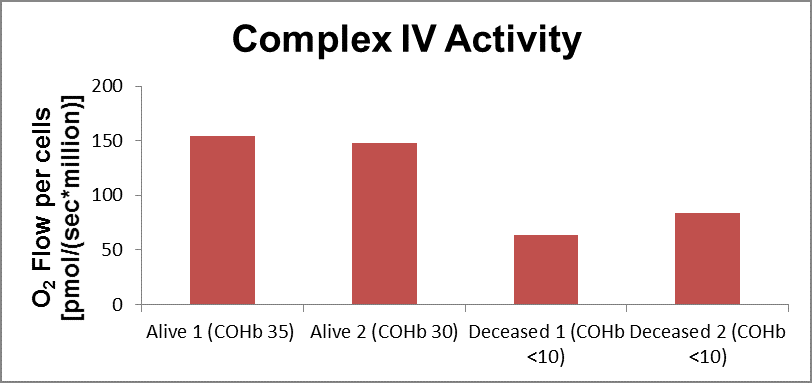
Specific CIV activity was obtained in frozen patient samples with the two-deceased patients showing decreased CIV activity when compared to the two alive patients
3. The measurement of mitochondrial respiration in human blood cells in sepsis
Mitochondrial dysfunction has been implicated in immune dysregulation and organ failure in sepsis but direct evidence of mitochondrial dysfunction in sepsis-related organ failure is limited. Mitochondrial dysfunction is present in circulating immune cells and is thought to be associated with duration of organ failure in sepsis. The following figure illustrates the decrease in the key parameters of mitochondrial respiration when compared to controls (not showed).