Perelman School of Medicine at the University of Pennsylvania
? Need help? Please feel free to e-mail us for more info.
People
Radhika Goenka, Post-doctoral Research Fellow
rgoenka@mail.med.upenn.edu
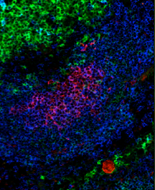
Radhika’s project focuses on understanding the regulation of BLyS family members during germinal center (GC) responses. While GC B cells are unable to bind BLyS in vivo, germinal center T cells provide a localized source of BLyS in the GC light zone. The paucity of BLyS in the GC correlates with the down-regulation of TACI receptors on GC B cells compared to naive follicular B cells. Thus, by making BLyS a 'rare commodity' in the GC presumably increases selection stringency during affinity maturation.
Yi Hao, Post-doctoral Research Fellow
hao@mail.med.upenn.edu
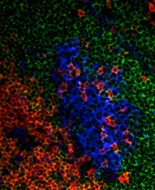
Yi's work is focused on the changes of B cell homeostasis in the peripheral pool with aging. Advancing age is associated with compromised immune responses. Yi has discovered a novel mature B cell population that accumulates with age. These cells are refractory to BCR and CD40 stimulation, but are responsive to either toll like receptor (TLR) -9 or -7. This shift in functional B cell subset composition may account for the diminished capacity to mount adaptive humoral responses in aged mice. The molecular basis mechanisms to select this subset is to be determined.
Martin Naradikian, Ph.D. Candidate in Immunology smartin@mail.med.upenn.edu
Patrick O’Neill, Research Specialist
oneilpat@mail.med.upenn.edu
Patrick is currently involved in collaborative work that involves modeling B lymphoablative therapies, BLyS receptor expression and anti-dsDNA analysis of B cell subsets in aged and lupus prone mice, in vitro analysis of a novel B cell population in aged mice, and the role of TACI and TFH cytokines in the germinal center.
Michael Oropallo, Ph.D. Candidate in Immunology
mant@mail.med.upenn.edu
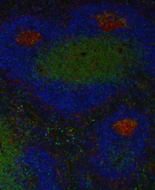
Mike is currently investigating how Rheumatoid Factor B cells become activated and escape tolerance. He has observed that after stimulation, RF B cells undergo a proliferation associated cell death. However, the addition of BLyS rescues this apoptosis through an unknown molecular mechanism.
Will J. Quinn III, Ph.D.
wquinn@mail.med.upenn.edu
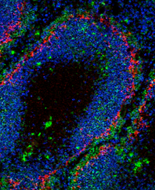
Plasma cells secrete antibodies. Two categories of plasma cells, short-and long-lived, are defined based on lifespan. Long-lived plasma cells are important because they secrete antibody indefinitely after antigen challenge, thereby maintaining antibody titers. We have found that plasma cells generated during thymus-dependent immune responses uniquely express RANKL. Further, we found that plasma cell-intrinsic RANKL expression is necessary for long-lived plasma cell generation and maintenance in vivo. This necessity is likely due in part to the ability of RANKL to induce myeloid cell APRIL production.
Jean Labriola Scholz, Ph.D., Research Investigator
jeanl@mail.med.upenn.edu
Jean currently studies B ablative therapies in mouse models of lupus and is pursuing new projects in the control of BLyS receptor gene expression and B cell repertoire analyses.
Vishal Sindhava, Postdoctoral Research Fellow sindhava@mail.med.upenn.edu
Vishal's work will focus on the mechanisms mediating long-lived plasma cell (LLPC) survival. The project will focus on testing the idea that RANK-L enables emerging LLPC to interact with myeloid cells to induce local, viability-promoting niches typified by APRIL production. Subsequently, we will determine whether RANK-L expression is necessary and/or sufficient for the establishment of LLPC populations.