Early Career Investigators
Lindsay W. Brubaker

Assistant Professor, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology
University of Colorado Anschutz Medical Campus
Medical School: George Washington University
OBGYN Residency: Stanford University
Fellowship in Gynecologic Oncology: University of Colorado
Award: Defining and targeting Chromobox2-mediated chemoresistance in high grade serous carcinoma
Research Description
I am a clinical and translational researcher, as well as a practicing Gynecologic Oncologist at the University of Colorado Anschutz Medical Campus. My research program seeks to define drivers of chemoresistance in high grade serous carcinoma, with the hope of identifying new opportunities for therapeutic development. Our current work is focused on the transcriptional repressor, polycomb repressor complex 1 (PRC1), and targeting one of its primary subunits, chromobox 2 (CBX2), which is associated with chemoresistance and worse survival. Our hope is that these investigations will translate to innovative clinical trial development and improved treatment options for our patients.
Elizabeth Christie, PhD

Elizabeth Christie, PhD Website
Group Leader in the Cancer Evolution and Metastasis Program at the Peter MacCallum Cancer Centre
Education: PhD, Ludwig Institute for Cancer Research and the University of Melbourne
Postdoctoral Training: Peter MacCallum Cancer, Victoria, Australia
Award: Improving Ovarian Cancer Outcomes by Understanding and Targeting Acquired Resistance
Research description
Acquired drug resistance, when a cancer initially responds well to treatment but upon disease recurrence it becomes progressively more resistant to treatment, is a major challenge that continues to lead to poor outcomes for individuals with high grade serous ovarian cancer (HGSOC).
The primary focus of my research program is to fully catalog the mechanisms of acquired drug resistance that arise in HGSOC by applying multi-omics to patient samples. We also seek to understand metastatic spread, develop blood- and tumor-based biomarkers of clinical response, and perform preclinical studies to identify novel treatment strategies for ovarian cancer.
Mohammed Dwidar, PhD

Assistant Professor, Department of Molecular Medicine, Cleveland Clinic Main Campus
Education: PhD Okinawa Institute of Science and Technology Graduate University, Onna, Japan
Award: Therapeutic Immunostimulatory Microbes for Treatment of Ovarian Cancer
Research Description
I am exploring the use of microbes as cancer therapeutics with special focus on ovarian and breast cancers. Additionally, my lab aims to create advanced synthetic biology tools and approaches that allow engineered bacteria to respond to the desired disease cues.
Shailendra K Dwivedi, PhD

Shailendra K Dwivedi, PhD Website
Assistant Professor
Dept. of OB/GYN (Gynecologic Oncology)
University of Oklahoma Health Science Center.
Oklahoma City, OK 73104
Education: Ph.D.: Biological Science, Jawaharlal Nehru University, New Delhi, India and CSIR-Central Drug Research Institute, Lucknow, India.
Postdoctoral Training: Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
Award: miR-195 Re-Expression: A Therapeutic Strategy for Chemo-Resistant Ovarian Cancer
Research Description
My research focuses on investigating the molecular signaling involved in ovarian cancer progression and discovering innovative therapeutic strategies. Specifically, I am exploring the significance of non-coding RNA in ovarian cancer and investigating its potential as a regulatory and therapeutic tool in cancer progression, drug resistance, metastasis, and the tumor microenvironment. To gain insights into the molecular pathways governed by non-coding RNAs, we use high-grade serous ovarian cancer cell lines grown as monolayer or anchorage-independent spheroids, patient-derived primary tumor cells, and ovarian cancer animal models. Our ultimate goal is to translate our research findings to improve the therapeutic outcomes of ovarian cancer patients.
Denarda Dangaj Laniti, PhD

Denarda Dangaj Laniti, PhD Website
Lecturer at the University of Lausanne and Group leader in the "Human Integrated Tumor Immunology Discovery Engine” or Hi-TIDE
Lausanne Branch of the Ludwig Institute for Cancer Research, affiliated with the Department of Oncology of Centre Hospitalier Universitaire Vaudois (CHUV).
Education
Undergraduate degree : Democritus University of Thrace, Greece
Graduate studies (PhD) : Democritus University of Thrace, Greece, and University of Pennsylvania, USA
Postdoctoral studies: Ludwig Institute for Cancer Research, University of Lausanne, Switzerland
Award: Understanding T cell senescence to optimize ovarian cancer immunotherapy
Research Description
My scientific group studies deeply the T cell responses and the tumor microenvironment of ovarian cancers and other solid tumors. Our research interests are to elucidate the evolution of ovarian cancer and its microenvironment with the overarching goal to improve immunotherapy.
Other research objectives are to gain a deep understanding of the molecular states of human TILs (tumor infiltrating lymphocytes) and the tumor microenvironment at stages of tumor escape or tumor rejection using systems immunology approaches. My lab studies tumor and stroma-driven exclusion mechanisms of TILs and also aims to understand how genomic alterations (ie homologous recombination repair deficiency, HRD) and tumor heterogeneity regulate TIL inflammation status of tumors.
We employ orthogonal approaches to discovery, including single cell proteomic, transcriptional, and epigenetic interrogation, advanced high dimensional microscopy, topologically referenced transcriptional profiling, 3D tumor organoids and histocultures, mouse models and computational tools.
Jessica Lang, PhD

Assistant Professor, Center for Human Genomics & Precision Medicine, Department of Pathology & Laboratory Medicine
University of Wisconsin-Madison
Education: PhD University of Wisconsin – Madison
Award: Living Models of Ovarian Cancer for Epigenetic Drug Screening and Mitochondrial Dysfunction
Research Description:
The goal of my lab is to incorporate precision medicine to improve treatment outcomes for ovarian cancer patients. Almost all high-grade serous ovarian carcinoma (HGSOC) patients still receive platinum-based chemotherapy treatment, with little known ahead of time on whether they will respond to treatment, other than their response to previous lines of platinum-based chemotherapy. We have been developing HGSOC organoids and associated assays to be able to multi-omically profile improved translational models of ovarian cancer. This includes application of whole exome sequencing, RNA-sequencing, CUT&Tag, and ATAC-seq to have an expanded set of predictive genomic and epigenetic features to associate with both clinical and organoid response to current standard of care and to test investigational epigenetic inhibitors. In addition, we have uncovered a potential mechanism of platinum-based chemotherapy resistance that has some basis for contributing to resistance through alteration of endogenous DNA damage. This hypothesis is an exciting new mechanistic study that has not been previously explored in ovarian cancer.
Alexander Melamed, MD, MPH

Alexander Melamed, MD, MPH Website
Associate Professor of Obstetrics, Gynecology and Reproductive Biology
Harvard Medical School
Ob/ Gyn Residency: Brigham and Women's Hospital and Massachusetts General Hospital
Gynecologic Oncology Fellowship: Massachusetts General Hospital
Award: Minimally invasive surgery for advanced ovarian cancer: a pilot randomized trial
Research Description
I am a health services and comparative effectiveness researcher and a practicing gynecologic oncologist at Massachusetts General Hospital. My research focuses on evaluating the effectiveness of healthcare services, with a focus on the role of surgery in improving outcomes among women with gynecologic cancer. To this end, I have taken a broad interdisciplinary approach incorporating translational, observational, and interventional clinical research. Much of my work seeks to estimate causal association in observational data. To that end, I have developed expertise in applying advanced analytic techniques to questions with clinical and policy significance in cancer care. My work has been published in high-impact medical journals including the New England Journal of Medicine, JAMA, and BMJ.
Dimitrios Nasioudis

Assistant Professor
Obstetrics and Gynecology at the Hospital of the University of Pennsylvania
Medical School: Aristotelian University of Thessaloniki
Residency: Hospital of the University of Pennsylvania
Fellowship: Hospital of the University of Pennsylvania
Award: Exploiting absence of TP53 mutations in epithelial ovarian cancer with next-generation p53 stabilizing agents.
Achuth Padmanabhan, PhD

Achuth Padmanabhan, PhD Website
Assistant Professor, UMBC, University of Maryland, Baltimore, MD
Assistant Professor, Department of Biological Sciences, University of Maryland Baltimore County;
Member, University of Maryland Marlene and Stewart Greenbaum Comprehensive Cancer Center.
Education: Ph.D., Biological Sciences, University of Maryland Baltimore County
Postdoctoral Training: Department of Molecular Biophysics and Biochemistry, Yale University
Department of Molecular and Cellular Biology, Baylor College of Medicine
Award: Role and Regulation of ZNF217 in Ovarian Cancer Metastasis and Drug Resistance
Research Description
The long-term goal of my lab is to identify factors that drive ovarian cancer progression and metastasis and develop clinically translatable strategies to target them. We are particularly interested in understanding the dynamic interaction between cancer cell intrinsic and extrinsic factors and their impact on tumor progression and therapeutic response.
Rebecca L. Porter, MD, PhD

Rebecca L. Porter, MD, PhD Website
Assistant Professor of Medicine, Harvard Medical School
Education: MD and PhD from the University of Rochester Medical Center
Award: Enhancing the Antitumor Activity of Natural Killer Cells in Ovarian Cancer
Research Description
I am a clinical and translational researcher and a practicing Medical Oncologist in the Gynecologic Oncology Program at the Dana-Farber Cancer Institute. My research aims to improve anti-tumor immune responses in patients with ovarian and other gynecologic cancers by developing and translating novel immunotherapeutic approaches into clinical practice, and understanding biomarkers of response and mechanisms driving immune escape to these therapies. These approaches include highly engineered CAR natural killer (NK) cell and T cell therapies as well as non-cellular systemic immunotherapy combinations.
Other website: Rebecca Porter | Harvard Catalyst Profiles | Harvard Catalyst
Rinda T. Soong, MD, PhD

Rinda T. Soong, MD, PhD Website
Assistant Professor, Department of Pathology, University of Pittsburgh School of Medicine
Education: MD, Johns Hopkins University School of Medicine
PhD and MPH, Johns Hopkins Bloomberg School of Public Health
Pathology residency and fellowship: Brigham and Women’s Hospital, Harvard Medical School
Award: Tubal Immune Milieu and Tumor Precursor Evolution in the Development of High-Grade Serous Carcinoma
Research description
The long-term goal of my research program is to identify factors that facilitate the initiation and progression of ovarian high grade serous carcinoma in relation to the immune microenvironment and host epidemiologic profiles. Our work interrogates human tissue, cell lines, and mouse models to examine the evolution of tumor precursors into malignancy, as well as the mechanisms underlying the heterogeneity and progression of carcinoma with the ultimate aims to uncover early detection biomarkers and therapeutic targets.
Priyanka Verma, PhD

Assistant Professor, Siteman Cancer Center, Washington University School of Medicine, St. Louis, MO
Education: Ph.D., Biochemistry, National Institute of Immunology, New Delhi, India
Postdoc: Department of Cancer Biology, University of Pennsylvania School of Medicine, Philadelphia, PA
Award: Replication Driven Vulnerabilities in Cyclin E Overexpressing Ovarian Cancers
Research Description
The overarching goal of the research in my lab is to understand how alterations in DNA replication and repair pathways impact etiology and chemotherapeutic responses in high-grade serous ovarian carcinoma. Our work integrates several molecular, cellular and functional genomic approaches to translate fundamental mechanistic discoveries into clinically impactful results.
Nan Zhang, PhD

Assistant Professor, Immunology, Microenvironment and Metastasis Program, Ellen and Ronald Caplan Cancer Center
Education: PhD, Biochemistry and Molecular Biology, University of Oklahoma Health Sciences Center
Award: Targeting resident macrophages to treat metastatic ovarian cancer
Research description
The Zhang lab focuses on the role of myeloid cells, including neutrophils and macrophages, in metastatic ovarian cancer. The goal is to find novel myeloid cell-based immunotherapies to treat or cure ovarian cancer in combination with immune checkpoint therapies or chemotherapy. Currently, we focus on treating metastatic ovarian cancer by activating myeloid cells using clinically relevant syngeneic mouse models and humanized mouse models. We use genetic mouse models, flow cytometry, ELISA, multiple types of fluorescence imaging (including multiphoton intravital imaging), single-cell RNA sequencing, spatial transcriptomics, proteomics, metabolomics, bioinformatics, and patient samples.
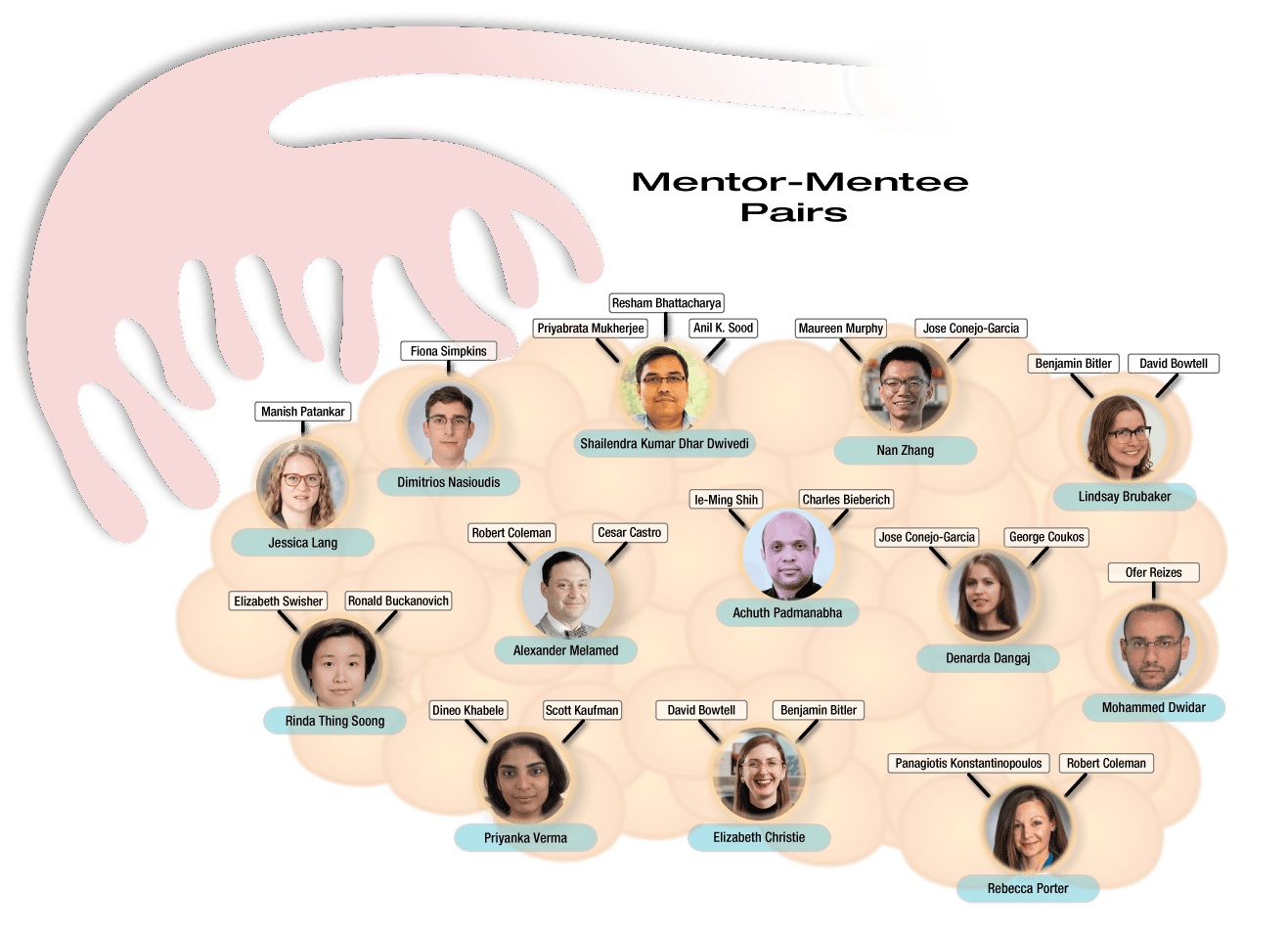
Full List of Mentors and Mentees:
|
Mentor: Maureen Murphy and Jose Conejo-Garcia |
Mentor: David Bowtell and Benjamin Bitler Mentee: Elizabeth Christie |
| Mentor: Benjamin Bitler and David Bowtell Mentee: Lindsay Brubaker |
Mentor: Panagiotis Konstantinopoulos and Robert Colemon Mentee: Rebecca Porter |
| Mentor: Ofer Reizes Mentee: Mohammed Dwidar |
Mentor: Dineo Khabele and Scott Kaufman Mentee: Priyanka Verma |
| Mentor: Ie-Ming Shih and Charles Bieberich Mentee: Achuth Padmanabhan |
Mentor: Elizabeth Swisher and Ronald Buckanovich Mentee: Rinda Thing Soong |
| Mentor: Resham Bhattacharya, Priyabrata Mukherjee and Anil K. Sood Mentee: Shailendra Dwivedi |
Mentor: Robert Coleman and Cesar Castro Mentee: Alexander Melamed |
| Mentor: Jose Conejo-Garcia and George Coukos Mentee: Denarda Dangaj |
Mentor: Manish Patankar Mentee: Jessica Lang |
| Mentor: Fionna Simpkins Mentee: Dimitrios Nasioudis |

