Pathogenesis
While ovarian cancer encompasses a diverse group of malignancies, high-grade serous carcinoma (HGSC) is the most common, aggressive, and lethal subtype. Recent research from our group and others has fundamentally shifted the understanding of HGSC origins, demonstrating that a significant proportion of these cancers arise from the Fallopian tube epithelium (FTE), rather than the ovarian surface epithelium. Our studies have identified two key precursor lesions in the Fallopian tube that contribute to HGSC development: the p53 signature and serous tubal intraepithelial carcinoma (STIC). These findings have been instrumental in redefining the early events in serous tumorigenesis.
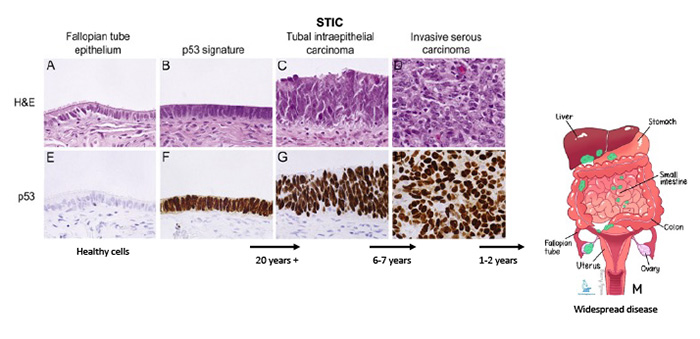
To advance our understanding of this progression, our lab has developed a suite of innovative model systems. These include an ex vivo model of benign FTE, Fallopian tube secretory cell lines to explore mechanism of transformation, genetically engineered mouse models targeting the FTE, and a library of patient-derived tumor xenograft (PDX) models that faithfully preserve the phenotypic and genotypic properties of the original tumors. By integrating insights from comprehensive genomic studies with these advanced models, we aim to identify key molecular drivers of serous tumorigenesis. This work seeks to unlock new targets for cancer interception and develop strategies for early detection, ultimately transforming the outlook for patients with HGSC.
This paradigm shift also has far-reaching implications for clinical practice. Our work has contributed to the adoption of opportunistic salpingectomy as a preventive strategy in average-risk women undergoing surgery for benign gynecologic conditions. Additionally, through ongoing clinical trials, we are exploring whether this approach provides comparable risk reduction for BRCA1/2 mutation carriers, potentially reshaping the standard of care for these high-risk individuals.

