Research
Research in the Luo Lab is focused on understanding how the somatosensory system functions. Somatosensation gives us the ability to respond to mechanical force or temperature, and allows us to feel sensations such as touch, pain, and itch. Without somatosensory neurons, we would be unable sense a hug or a kiss, a summer breeze, or distinguish between different textures. We would also be unaware of pain, which serves to protect us from potential bodily harm. Touch and pain are among the least understood sensory modalities at the molecular and cellular level in mammals, and at present, deducing which types of somatosensory neurons are responsible for different forms of touch sensations is one of the biggest puzzles in sensory neuroscience. Furthermore, understanding the changes in the somatosensory system that occur after pathological conditions arise represents another main goal in the lab. Thus, we study mechanisms underlying touch, pain, and itch at the single cell, circuit, and behavioral levels using a diverse array of techniques ranging from molecular biology and electrophysiology to optogenetics and cutting-edge behavioral analysis.
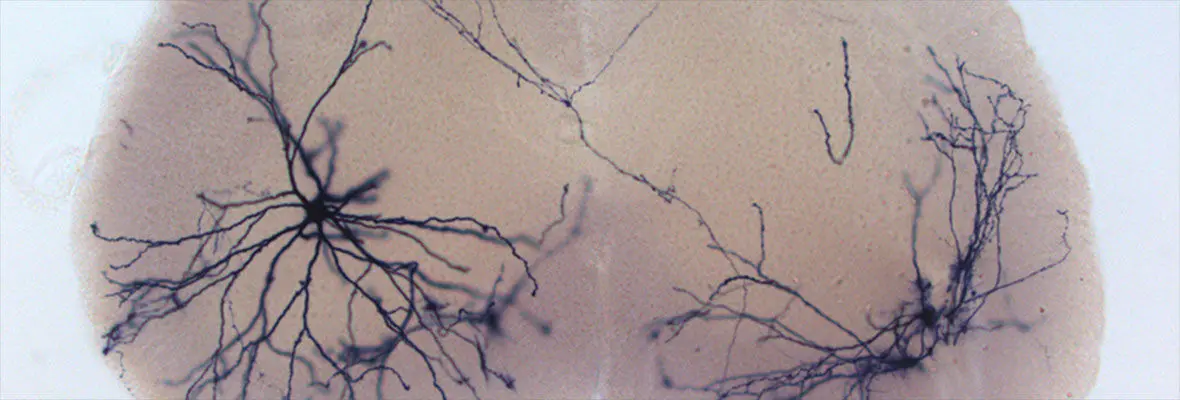
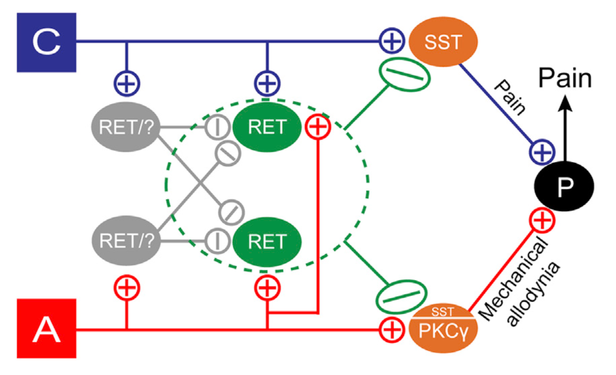
A major focus in the lab is understanding the sensation of light touch, and the crosstalk between touch and pain. Recently, our lab identified a population of deep layer spinal cord dorsal horn (dDH) inhibitory interneurons that expresses the receptor tyrosine kinase Ret (Cui et al., Neuron 2016). We found that these neurons negatively regulate dorsal horn pain and touch pathways through pre- and postsynaptic inhibition, and are involved in the dampening of basal pain as well as both inflammatory and neuropathic pain. We proposed that these interneurons act as gating neurons to modulate pain transmission in the spinal cord (Figure 1), thus supporting the classic gate control theory of pain proposed by Melzack and Wall in 1965 which states that non-noxious inputs close “gates” in the spinal cord (via inhibitory interneurons) which suppress pain signals.
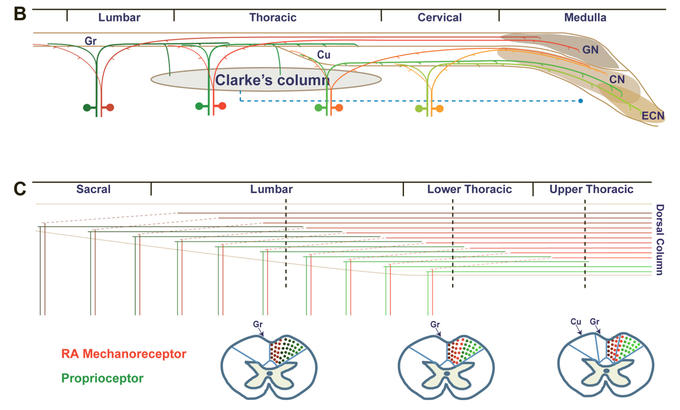
Earlier work using a novel genetic labeling technique (Luo et al., Neuron 2009) identified a population of somatosensory neurons that expresses Ret early on in development and develops into rapidly adapting (RA) mechanoreceptors that sense light touch. This ability to specifically label RA mechanoreceptors allowed us to demonstrate that axons from mechanosensory and proprioceptive (labelled using the marker parvalbumin) neurons ascend the spinal cord dorsal column in a way that is segregated by both modality and a somatotopic map (Niu et al., J. Neurosci. 2013; Figure 2). Further studies on Ret in the lab investigated the role of Ret in developing touch dome Merkel cells (Niu et al., PLoS One 2014), developing Pacinian corpuscles (Fleming et al., J. Neurosci. 2016), and cis/trans Ret signaling in sensory neuron development (Fleming et al., eLife 2015). The ability to specifically label RA mechanoreceptors via early expression of Ret will allow the lab to further investigate the role of these neurons in touch and pain behavior.
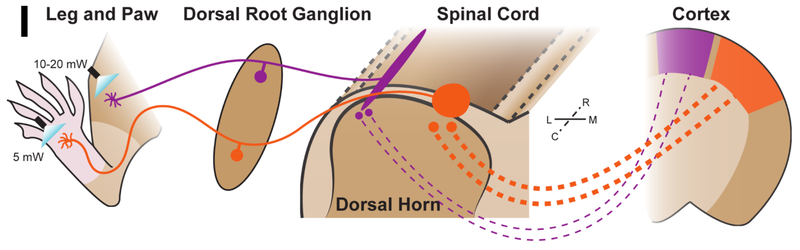
Our lab is also interested in the sensation of noxious and itchy stimuli through small diameter nociceptors. We developed a novel mouse line that allows us to examine the non-peptidergic population of nociceptors labelled by the G protein coupled receptor Mrgprd, and found that the central terminals of these nociceptors have distinct morphologies based their peripheral innervation in the paw vs. elsewhere on the body (e.g., the leg) (Olson et al., eLife 2017). Additional electrophysiological and behavioral experiments using optogenetics revealed that innervation from the paw shows greater sensitivity compared to innervation from the leg (Figure 3). Present work in the lab seeks to further understand the Mrgprd+ population of non-peptidergic neurons in the context of pain and itch, as well as mechanisms underlying the polymodal nature of these and other nociceptor populations in both normal and pathological conditions.
Additionally, the lab is currently developing new approaches for characterizing and differentiating among touch, pain, and itch behavior, and is using cutting-edge methods to improve definitions for a wide range of somatosensory behaviors. We seek to utilize these methods to guide future experiments for studying acute and chronic pain. We are also interested in new molecular techniques for linking behavior with different populations of somatosensory neurons, and investigating the circuits involved in touch, pain, and itch. Lastly, we continue to be interested in the development of the somatosensory system and the general architecture of the spinal cord.
Updated 10/2017