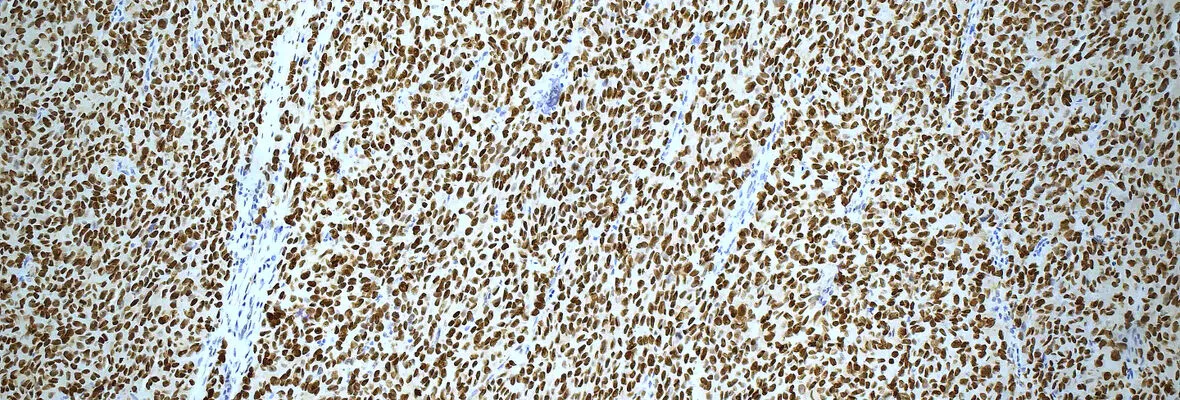
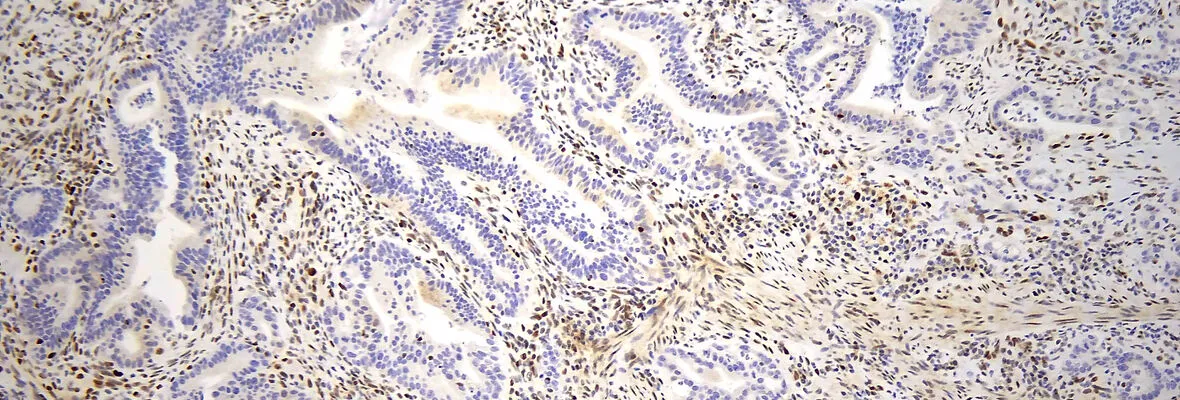
Immunohistochemistry and Immunofluorescence
Our laboratory offers a broad menu of clinically validated immunohistochemistry (IHC) and in situ hybridization (ISH) tests and provides expert support for the development and validation of new assays. With experience across multiple detection techniques, we tailor protocols to deliver high-quality, reproducible results.
The Pathology Clinical Service Center (PCSC) is equipped with both a Leica Bond III autostainer and the advanced Leica Bond Prime platform, facilitating the execution of high-throughput studies with minimal inter-assay variability. The PCSC works closely with the clinical IHC lab and its Medical Director to ensure the use of the most effective antibody clones and up-to-date assays.
The PCSC offers the following services:
- High-throughput staining for IHC, IF, and ISH, including RNAscope
- Optimization and validation of new IHC, IF, and ISH assays
- Single- and dual- IHC, IF, and ISH.*
The current estimated time for completion of new IHC/IF projects is 4 weeks.
*Multiplex assays (three or more colors), as well as multispectral imaging, are not presently available.