Finding a Cure for RVCL
Gene Therapy Update
 The Miner laboratory can now fully correct TREX1 mutations in cultured cells in the laboratory. Now the goal is to develop delivery systems, and to test the safety and efficacy of gene therapy in model systems.
The Miner laboratory can now fully correct TREX1 mutations in cultured cells in the laboratory. Now the goal is to develop delivery systems, and to test the safety and efficacy of gene therapy in model systems.
Developing Personalized Medicines for RVCL
We partner with patients, physicians, and scientists from around the world in an effort to develop personalized medicines for RVCL. Thanks to generous support from The Clayco Foundation, as well as from the families and friends of patients with RVCL, we are pushing forward faster than ever in this effort. Our team consists of a group of rheumatologists, neurologists, ophthalmologists, genetic counselors, and researchers, all with the singular goal of helping you and your family.
Click the menus below to learn more about pathways to develop precision and personalized medicine for patients RVCL.
Therapies currently in development
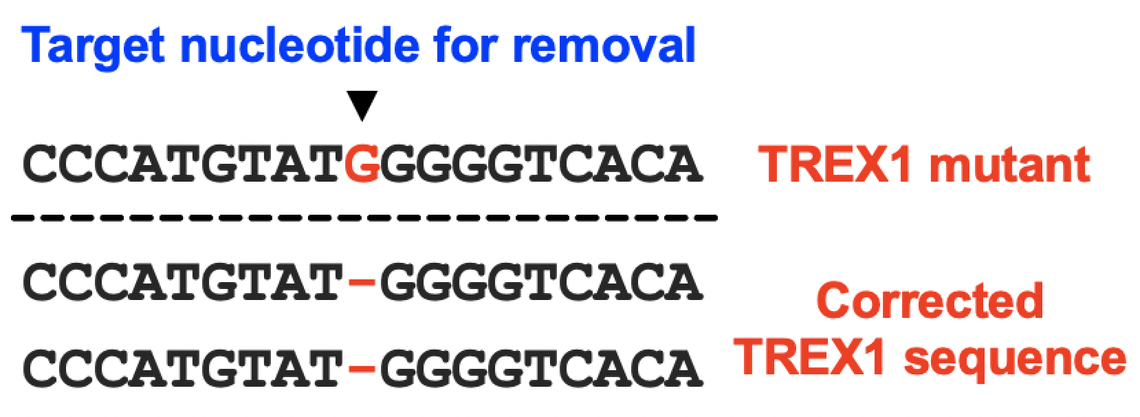
CRISPR/Cas9 is a gene editing technology that can delete a gene entirely in patients or, in some cases, to correct mutations in specific cell types. We are using a specialized form of CRISPR and collaborating with experts on delivery systems so that we can use CRISPR to treat RVCL by correcting the mutated TREX1 gene in patients with RVCL. This is an exciting potential treatment option, but using CRISPR to treat RVCL is no small task. Nevertheless, CRISPR is one of the key personalized medicine pathways that we are actively pursuing here at the RVCL Research Center at Penn.
Our laboratories are developing CRISPR-based editing technologies that will fully correct disease-causing mutations in the TREX1 gene, and we are hopeful that CRISPR-based therapies will eventually become available for the treatment RVCL.
What is gene therapy?
Scientists and doctors have been talking about gene therapy for decades. Now, gene therapy is becoming a reality for rare diseases, but this type of therapy must be conducted carefully, ethically, and with rigorous testing. One major challenge for RVCL will be delivering gene therapies to the correct cell types, but Penn Medicine has experience developing and testing delivery systems for genetic diseases. You can learn more about the vital role that Penn Medicine played in the development of gene therapy in this video from the Howard Hughes Medical Institute. Note the major contributions of Penn Medicine physician-scientists Jean Bennett and Albert Maguire, a key member of our RVCL Research Center at Penn.
What are small molecule drugs?
Most pills are small molecular durgs. In contrast, antibody drugs are large protein molecules that must be administered by injection. Gene editing drugs also are very complicated to deliver.
Small molecular inhibitors are less precise than antibody drugs and gene therapies, but they still have several major advantages. One major advantage of small molecular inhibitors is that they are easier to produce, and they can target many cell types throughout the body very easily. Dr. Nouri Neamati at the University of Michigan is sending compounds to the Miner laboratory for validation and additional testing. The Miner laboratory has generated multiple model systems for the testing of therapies for RVCL.
Clinical Trials
What is crizanlizumab?
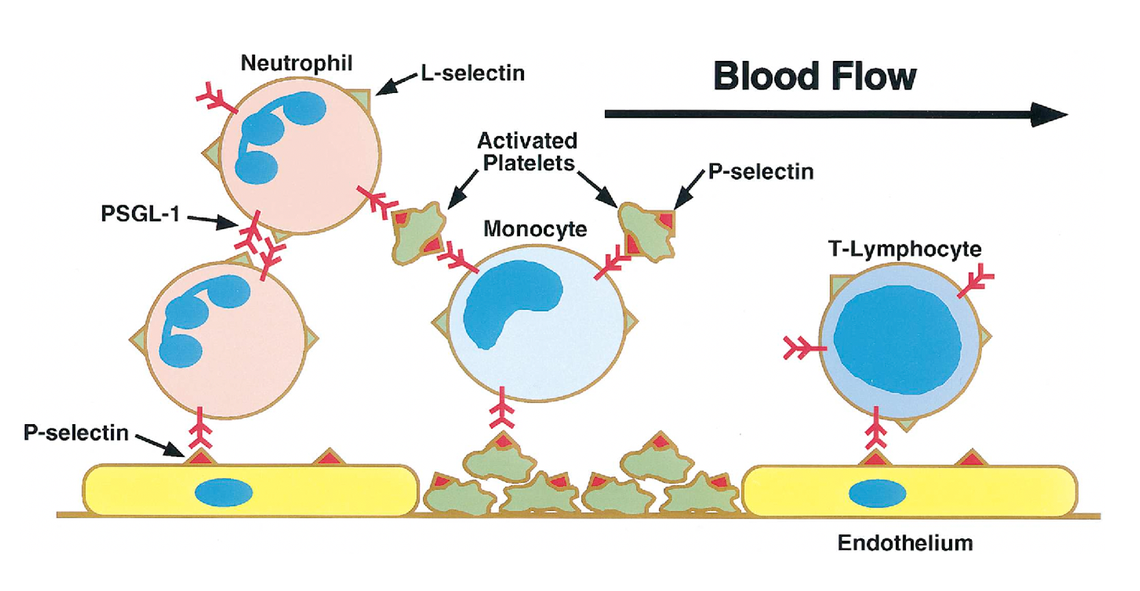
Our previous clinical trial tests whether a drug called crizanlizumab slows disease progression in patients with RVCL. Crizanlizumab is a human antibody drug that blocks a protein called P-selectin. P-selectin is a protein that is normally displayed on the blood vessel wall. The normal function of P-selectin is to capture white blood cells so that they attach to the vessel wall, as shown in the video below. Capturing white blood cells is a good thing during inflammation. However, in certain diseases, the blood vessel wall is damaged and P-selectin can promote disease-associated blockages of blood vessels, leading to organ damage and injury.
Antibody drugs are commonly used in many diseases. Naturally occuring antibodies are produced by the immune system to protect the body against germs. An antibody drug is a human antibody designed to block the function of one of our own proteins, with the goal of controlling disease.
Blocking P-selectin can prevent white blood cells from obstructing blood flow. Crizanlizumab, the human antibody that blocks P-selectin, is already FDA-approved to prevent painful crises in sickle cell disease, another rare disease associated with obstruction of small blood vessels. In the video on the right, you can observe white blood cells rolling along the blood vessel wall.
When crizanlizumab is administered, P-selectin is blocked, and white blood cells no longer adhere to the wall of the blood vessel. This can improve blood flow when white blood cells contribute to blockages, but whether crizanlizumab is helpful in the treatment of RVCL is unclear.
Beginning in 2016, there was a phase 1 clinical trial of aclarubicin for RVCL conducted by Drs. John Atkinson and Jonathan Miner. Aclarubicin is no longer being pursued as a potential therapy for RVCL. Nevertheless, the aclarubicin trial was a key step toward future clinical trials. Now, there is a much larger Phase 2 clinical trial of a drug called crizanlizumab, a medication that is already FDA-approved for the treatment of another disease.
Bone marrow transplant involves the replacement of bone marrow and blood cells with donor cells. Since the donor cells would not have the TREX1 mutation, the new blood cells that develop after transplantation would no longer have the RVCL-causing mutation in TREX1. Bone marrow transplant is a very high-risk procedure, which is usually reserved for the treatment of certain cancers (e.g., leukemia or multiple myeloma). Bone marrow transplant creates significant risk of infection and organ damage. No RVCL patient has ever undergone bone marrow transplant, and we do not know whether this treatment approach would be effective for RVCL or not. However, if RVCL is caused by effects of the mutant TREX1 in blood cells, then this may become a viable treatment option in the future. Using model systems in the laboratory, research is currently underway to determine whether mutant TREX1 in blood cells might be responsible for the disease. Please reach out to us if you'd like to learn more.
Prevention of RVCL
What is preimplantation genetic testing?
Some couples may choose to perform in vitro fertilization followed by preimplantation genetic testing. This allows patients to select an embryos that do not have the TREX1 mutation that causes RVCL. The video below demonstrates the process of pre pre-implantation (day 3) embryo biopsy, from an 8-cell embryo. Embryos tolerate removal of cells for genetic testing.
Precision Medicine
 Learn more by exploring these links:
Learn more by exploring these links:
 If you're interested in learning more about the RVCL Research Center at Penn, or if you're interested in finding ways to support our work, please reach out to us directly via email.
If you're interested in learning more about the RVCL Research Center at Penn, or if you're interested in finding ways to support our work, please reach out to us directly via email.

