Research Platforms
Research Platforms: The Susztak Lab Discovery Ecosystem
The Susztak Lab has engineered a synergistic set of research platforms that enable large-scale, hypothesis-driven discovery directly in human kidney disease. These platforms integrate deep molecular resolution with longitudinal clinical outcomes and AI-enabled synthesis, providing a foundation for all scientific programs in the laboratory.
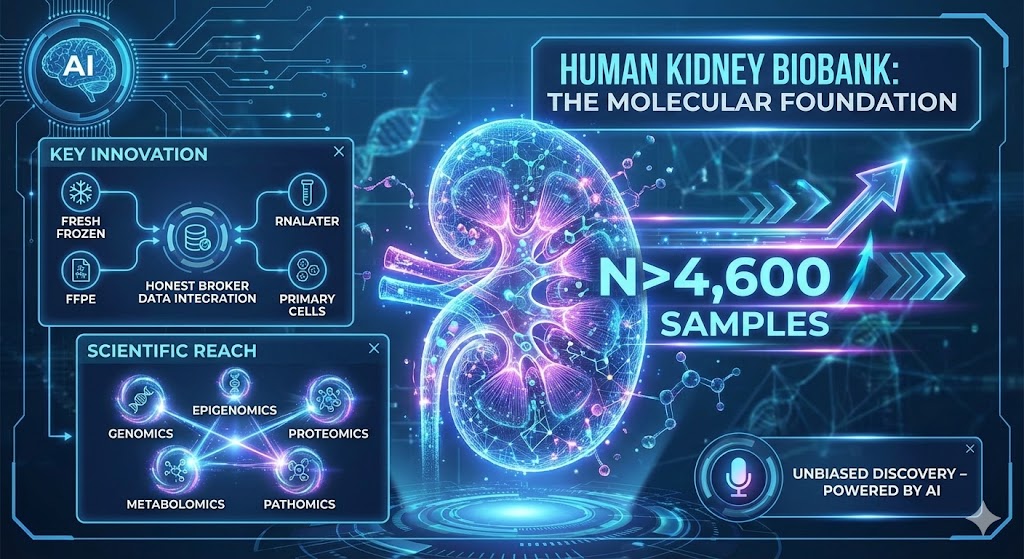
1. Human Kidney Biobank: The Molecular Foundation
The Power: This platform overcomes the "scarcity bottleneck" in nephrology by providing one of the world's most deeply phenotyped human kidney repositories (n>4,600).
-
Key Innovation: We integrate "Honest Broker" curated clinical data with multi-modal preservation—including Fresh Frozen, FFPE, RNALater, and Primary Cell cultures. This enables the simultaneous study of kidney structure, cellular function, and underlying genetics from the same patient.
-
Scientific Reach: The biobank supports unbiased discovery across the entire molecular and structural spectrum: genomics, epigenomics, proteomics, metabolomics, and pathomics.
-
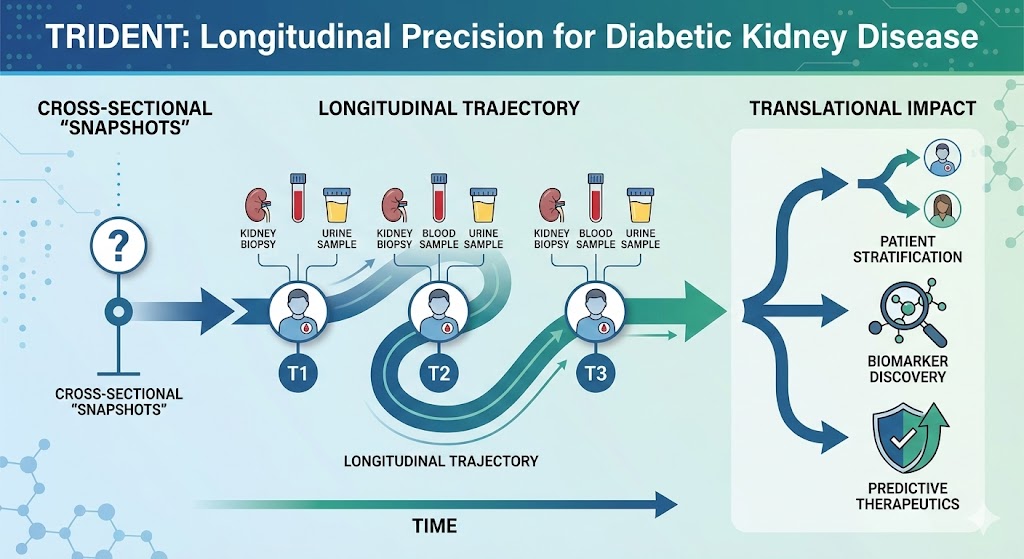
2. TRIDENT: Longitudinal Precision for Diabetes
-
The Power: Transformative Research in Diabetic Nephropathy (TRIDENT) moves beyond cross-sectional "snapshots" to capture the dynamic molecular trajectory of Diabetic Kidney Disease (DKD).
-
Deep Phenotyping: This multi-center, prospective cohort follows individuals with diabetes, pairing longitudinal tissue collection with non-invasive biofluids (urine and plasma).
-
Translational Impact: TRIDENT enables high-resolution patient stratification and the discovery of biomarkers that predict therapeutic response and disease progression long before clinical decline occurs.
-
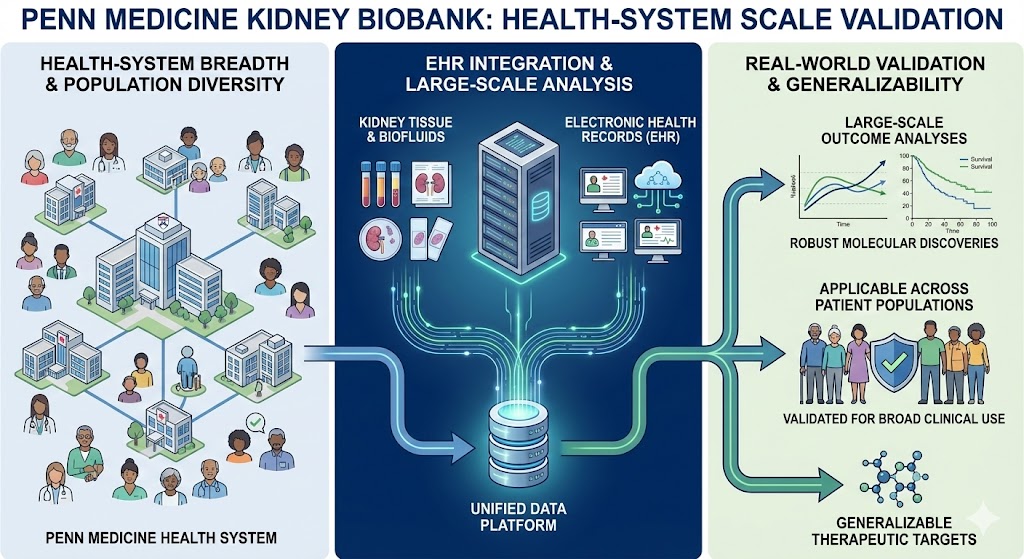
3. Penn Medicine Kidney Biobank: Health-System Scale Validation
-
The Power: This platform extends discovery beyond individual cohorts by providing health-system–scale breadth and population diversity.
-
EHR Integration: By linking kidney tissue and biofluids to Electronic Health Record (EHR) data across the entire Penn Medicine system, we enable large-scale outcome analyses and real-world clinical validation.
-
Generalizability: This scale ensures that molecular discoveries are robust and applicable across a broad, diverse patient population and various stages of kidney disease.
-
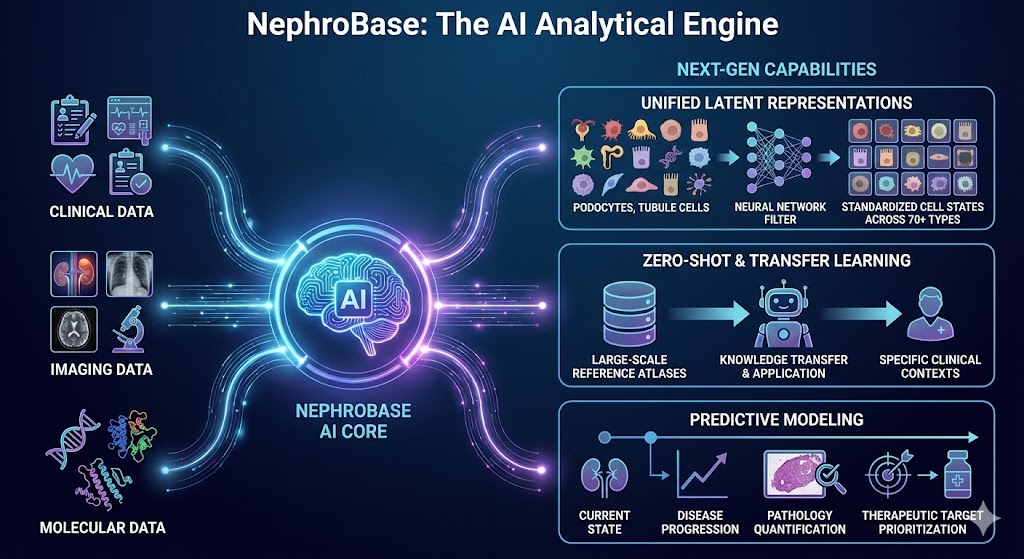
4. NephroBase: The AI Analytical Engine
-
The Power: NephroBase is our kidney-specific computational and AI platform that serves as the analytical "brain," connecting clinical, imaging, and molecular data across all other platforms.
-
Next-Gen Capabilities:
-
Unified Latent Representations: Standardizing cell states across 70+ kidney cell types to enable seamless cross-platform integration.
-
Zero-Shot & Transfer Learning: Utilizing AI to apply knowledge from large-scale reference atlases to specific clinical contexts.
-
Predictive Modeling: Moving from description to automated prediction of disease progression, pathology quantification, and therapeutic target prioritization.
-
-
