Publications
Featured Publications
Immunity (2005)
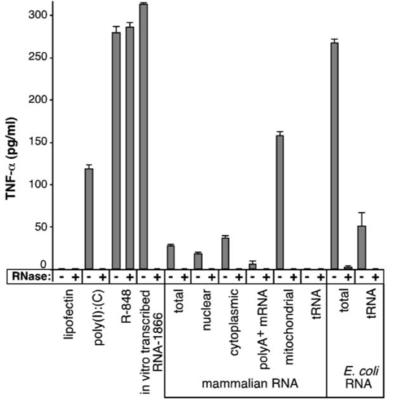
DNA and RNA stimulate the mammalian innate immune system through activation of Toll-like receptors (TLRs). DNA containing methylated CpG motifs, however, is not stimulatory. Selected nucleosides in naturally occurring RNA are also methylated or otherwise modified, but the immunomodulatory effects of these alterations remain untested. We show that RNA signals through human TLR3, TLR7, and TLR8, but incorporation of modified nucleosides m5C, m6A, m5U, s2U, or pseudouridine ablates activity. Dendritic cells (DCs) exposed to such modified RNA express significantly less cytokines and activation markers than those treated with unmodified RNA. DCs and TLR-expressing cells are potently activated by bacterial and mitochondrial RNA, but not by mammalian total RNA, which is abundant in modified nucleosides. We conclude that nucleoside modifications suppress the potential of RNA to activate DCs. The innate immune system may therefore detect RNA lacking nucleoside modification as a means of selectively responding to bacteria or necrotic tissue.
PLoS Pathogens (2020)
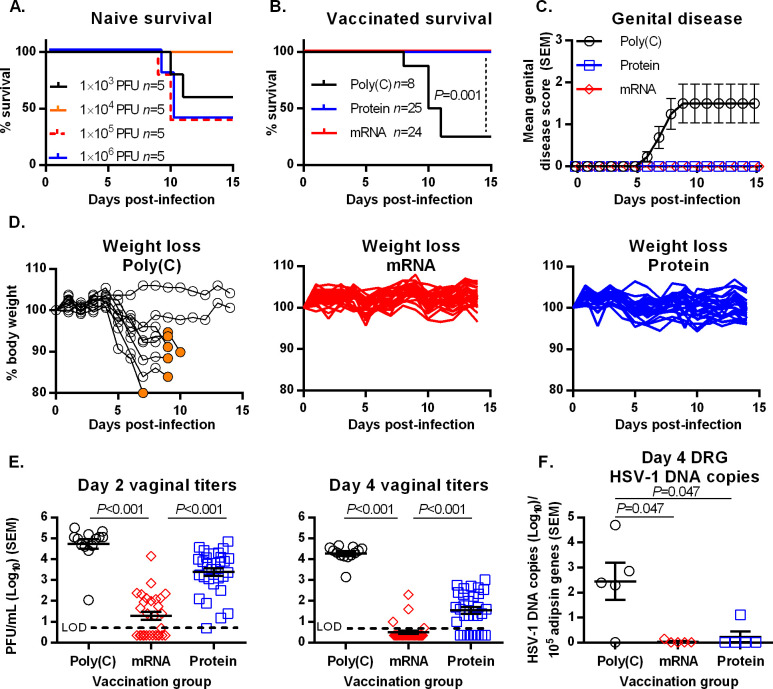
HSV-1 causes 50% of first-time genital herpes infections in resource-rich countries and affects 190 million people worldwide. A prophylactic herpes vaccine is needed to protect against genital infections by both HSV-1 and HSV-2. Vaccinated mice were totally protected against death, genital disease and infection of dorsal root ganglia caused by both viruses, but somewhat better protected against vaginal titers after HSV-2 infection.
Immunity (2020)
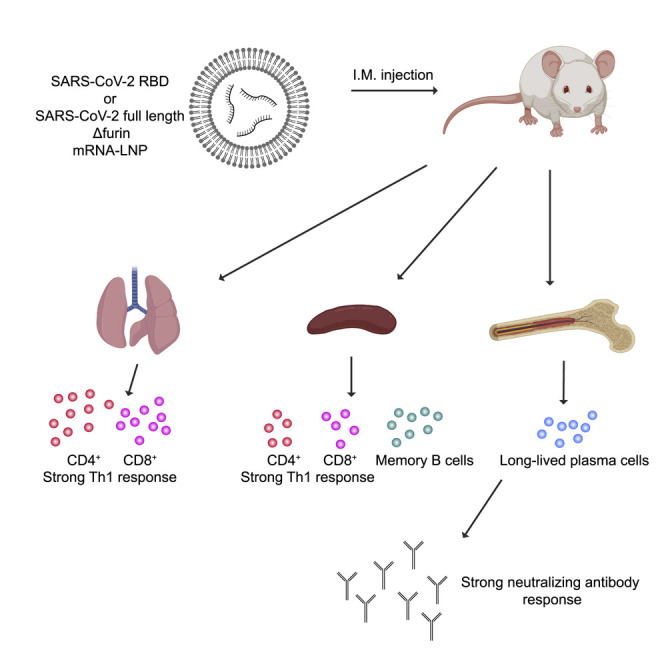
Here, we provide a detailed evaluation of the immunogenicity of lipid nanoparticle-encapsulated, nucleoside-modified mRNA (mRNA-LNP) vaccines encoding the full-length SARS-CoV-2 spike protein or the spike receptor binding domain in mice. We demonstrate that a single dose of these vaccines induces strong type 1 CD4+ and CD8+ T cell responses, as well as long-lived plasma and memory B cell responses. Additionally, we detect robust and sustained neutralizing antibody responses and the antibodies elicited by nucleoside-modified mRNA vaccines do not show antibody-dependent enhancement of infection in vitro. Our findings suggest that the nucleoside-modified mRNA-LNP vaccine platform can induce robust immune responses and is a promising candidate to combat COVID-19.
Cell Host Microbe (2021)
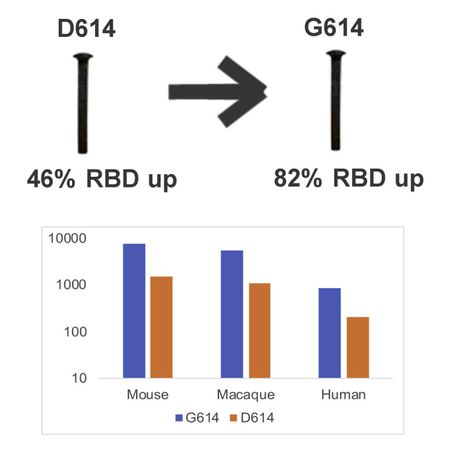
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein acquired a D614G mutation early in the pandemic that confers greater infectivity and is now the globally dominant form. To determine whether D614G might also mediate neutralization escape that could compromise vaccine efficacy, sera from spike-immunized mice, nonhuman primates, and humans were evaluated for neutralization of pseudoviruses bearing either D614 or G614 spike. In all cases, the G614 pseudovirus was moderately more susceptible to neutralization. The G614 pseudovirus also was more susceptible to neutralization by receptor-binding domain (RBD) monoclonal antibodies and convalescent sera from people infected with either form of the virus. Negative stain electron microscopy revealed a higher percentage of the 1-RBD "up" conformation in the G614 spike, suggesting increased epitope exposure as a mechanism of enhanced vulnerability to neutralization. Based on these findings, the D614G mutation is not expected to be an obstacle for current vaccine development.
Science Advances (2021)
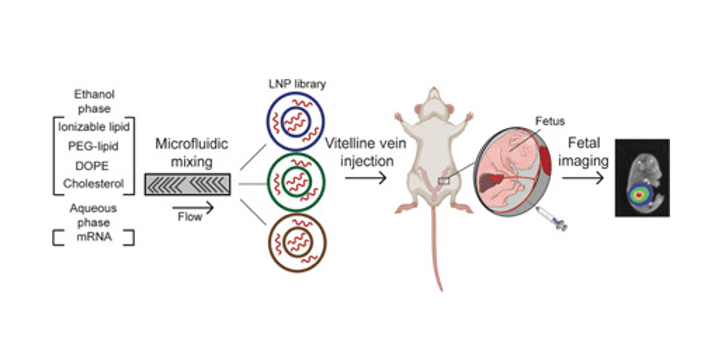
Clinical advances enable the prenatal diagnosis of genetic diseases that are candidates for gene and enzyme therapies such as messenger RNA (mRNA)-mediated protein replacement. Prenatal mRNA therapies can treat disease before the onset of irreversible pathology with high therapeutic efficacy and safety due to the small fetal size, immature immune system, and abundance of progenitor cells. However, the development of nonviral platforms for prenatal delivery is nascent. We developed a library of ionizable lipid nanoparticles (LNPs) for in utero mRNA delivery to mouse fetuses. We screened LNPs for luciferase mRNA delivery and identified formulations that accumulate within fetal livers, lungs, and intestines with higher efficiency and safety compared to benchmark delivery systems, DLin-MC3-DMA and jetPEI. We demonstrate that LNPs can deliver mRNAs to induce hepatic production of therapeutic secreted proteins. These LNPs may provide a platform for in utero mRNA delivery for protein replacement and gene editing.
All Publications
-
Macrophage cell therapy enabled by interleukin-4 mRNA-loaded lipid nanoparticles to sustain a pro-reparative phenotype in inflammatory injuries.
O'Brien EM, Tylek T, Geisler HC, Mukalel AJ, Whitaker RC, Sung S, Binder-Markey BI, Weissman D, Mitchell MJ, Spiller KL. Biomaterials. 2026 May;328:123869 Epub 2025 Nov 25 -
Neonatal mice immune response to COVID-19 mRNA vaccine.
Lotspeich-Cole L, Jha MK, Parvathaneni S, Lee RC, Weissman D, Major M, Akkoyunlu M. Vaccine. 2026 Mar 7;75:128271 Epub 2026 Jan 28 -
mRNA vaccines targeting Leptospira immunoglobulin-like proteins confer partial protection in a hamster model of leptospirosis.
Techawiwattanaboon T, Leekitcharoenphon R, Alameh MG, Boonkea S, Sangkanjanavanich N, Nakornpakdee Y, Ajimathorn Y, Prompetchara E, Ketloy C, Buranapraditkun S, Palaga T, Kanthawong S, Heyes J, Weissman D, Ruxrungtham K, Patarakul K. Vaccine. 2026 Feb 15;73:128099 Epub 2025 Dec 25 -
Targeted Lipid Nanoparticle Delivery of FAP-CAR mRNA Enables Potent In Vivo T-Cell Engineering Against Pancreatic Tumors.
Bajbouj K, Xiao Z, Todd L, Huang L, Papp TE, Halilovic F, Ramani J, Bao Y, Butcher M, Bot A, Aghajanian H, June CH, Weissman D, Parhiz H, Albelda SM, Pure E. Cancer immunology research. 2026 Feb 13 -
In vivo chimeric antigen receptor (CAR)-T cell therapy.
Bot A, Scharenberg A, Friedman K, Guey L, Hofmeister R, Andorko JI, Klichinsky M, Neumann F, Shah JV, Swayer AJ, Trudeau K, Weissman D, Stephan MT, Buchholz CJ, June CH. Nature reviews. Drug discovery. 2026 Feb;25(2):116-137 Epub 2025 Sep 30 -
Preclinical evaluation of DENV1 mRNA vaccines in mice: Toward improved neutralizing antibody and T cell responses.
Khawsang C, Prompetchara E, Saithong S, Tharakhet K, Kaewpang P, Yostrerat N, Wangsoontorn P, Pitakpolrat P, Buranapraditkun S, Puttikhunt C, Lam K, Heyes J, Ketloy C, Weissman D, Ruxrungtham K. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2026 Feb;195:119037 Epub 2026 Jan 23 -
High-Throughput In Vivo Screening Using Barcoded mRNA Identifies Lipid Nanoparticles With Extrahepatic Tropism for In Situ Immunoengineering.
Hamilton AG, Thatte AS, Xu J, Luo Z, Safford HC, Swingle KL, Muscat-Rivera J, Kegel M, Han X, Joseph RA, Murray AM, Geisler HC, Whitaker RC, Xue L, Spektor R, Melamed JR, Weissman D, Mitchell MJ. Advanced materials (Deerfield Beach, Fla.). 2026 Jan 28;:e14370 -
Targeting DNA-LNPs to Endothelial Cells Improves Expression Magnitude, Duration, and Specificity.
Marzolini N, Brysgel TV, Rahman RJ, Essien EO, Nwe SY, Wu J, Majumder A, Patel MN, Tiwari S, Espy CL, Dong F, Santos-De León SA, Shah A, Shuvaev VV, Hood ED, Chase LS, Weissman D, Katzen JB, Frank DB, Bennett ML, Marcos-Contreras OA, Myerson JW, Muzykantov VR, Reyes-Esteves S, Brenner JS. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2026 Jan 20;:e15480 -
An influenza HA mRNA-LNP vaccine induces potent responses in newborn nonhuman primates that enhance protection from challenge.
Page CL, Holbrook BC, Crofts KF, Alameh MG, Davis B, Caudell D, Weissman D, Alexander-Miller MA. NPJ vaccines. 2025 Dec 26;11(1):2 -
An integrated workflow for structural virology with a 100 keV electron microscope.
Pathirage R, Dutta M, Parsons RJ, Lella M, Atwood E, Zhang QE, May A, Johnson A, Huang X, Flemming J, Kumar U, Marayati BF, Spurrier MA, Liu C, Zhuo J, Song K, Adhikari RD, Sammour S, Ilevbare V, Abram C, Diaz M, Guzman A, Rai J, Skelly AN, Hogarty MP, Anasti K, Purro M, Lindsay M, Alam M, Weissman D, Herschchorn A, Hahn BH, Shaw GM, Sharma A, Heaton NS, Edwards RJ, Henderson R, Denny T, Saunders KO, Siliciano J, Siliciano R, Haynes BF, Janowska K, Acharya P. bioRxiv : the preprint server for biology. 2025 Dec 10 -
Mannich reaction-based combinatorial libraries identify antioxidant ionizable lipids for mRNA delivery with reduced immunogenicity.
Gong N, Kim D, Alameh MG, El-Mayta R, Han EL, Dwivedi G, Palanki R, Shi Q, Han X, Xue L, Xu J, Meng Z, Luo T, Figueroa-Espada CG, Weissman D, Li J, Mitchell MJ. Nature biomedical engineering. 2025 Dec;9(12):2181-2195 Epub 2025 Jul 18 -
Author Correction: Mannich reaction-based combinatorial libraries identify antioxidant ionizable lipids for mRNA delivery with reduced immunogenicity.
Gong N, Kim D, Alameh MG, El-Mayta R, Han EL, Dwivedi G, Palanki R, Shi Q, Han X, Xue L, Xu J, Meng Z, Luo T, Figueroa-Espada CG, Weissman D, Li J, Mitchell MJ. Nature biomedical engineering. 2025 Dec;9(12):2215 -
SRSF3 knockdown-induced cellular senescence as a possible therapeutic strategy for non-small cell lung cancer.
Nakamichi S, von Muhlinen N, Yamada L, Melamed JR, Papp TE, Parhiz H, Weissman D, Horikawa I, Harris CC. Carcinogenesis. 2025 Nov 21;46(4) -
RNA chemistry and therapeutics.
Wang S, Weissman D, Dong Y. Nature reviews. Drug discovery. 2025 Nov;24(11):828-851 Epub 2025 Jul 14 -
Hepatitis C virus modified (S)E2(F442NYT)-mRNA-LNP candidate vaccine promotes helper CXCR5(+)T cells.
Haga Y, Babu E P, Swiderska-Syn M, Reagan EK, Weissman D, Ray R. Journal of virology. 2025 Oct 23;99(10):e0135525 Epub 2025 Sep 5 -
Anionic lipids modulate mRNA-lipid nanoparticle immunogenicity and confer protection in a mouse model of multiple sclerosis.
Melamed JR, Muscat-Rivera J, Kegel M, Chaboub LS, Perez-Tremble R, Bhalla NS, Ni H, Sun H, Weissman D. bioRxiv : the preprint server for biology. 2025 Oct 17 -
Lipid nanoparticle co-delivery of mRNA and a small molecule drug for oral cancer chemoimmunotherapy.
Padilla MS, Li JJ, Zhang Q, Patwari K, Shi S, Yamagata HM, Joseph RA, Hymms BN, Teerdhala SV, Alameh MG, Weissman D, Le AD, Mitchell MJ. bioRxiv : the preprint server for biology. 2025 Oct 6 -
Consistent Induction of Broadly Neutralizing HIV Antibodies by a Novel Two-Step Mechanism Informs Immunogen Design.
Skelly AN, Gristick HB, Li H, Gavor E, Connell AJ, Kreider EF, Marchitto L, Hogarty MP, Newby ML, Allen JD, Liu W, West AP Jr, Ayyanathan K, Campion MS, Winters K, Gordon CG, Osbaldeston RA, Akeley MJ, Li Y, Singh A, Cruickshank K, Park Y, Zhao C, Li X, Amereh K, Itallie EV, Carey JW, Albertus A, DeLaitsch AT, Keeffe JR, Lituchy MG, Walsh AA, Morris DJ, Habib R, Bibollet-Ruche F, Mishra N, Avillion G, Koranda NS, Plante SJ, Martella CL, Lora J, Wang EJD, Lewis MG, Martin MA, Nussenzweig MC, Seaman MS, Irvine DJ, Wiehe KJ, Haynes BF, Wagh K, Korber BT, Andrabi R, Crispin M, Weissman D, Bjorkman PJ, Hahn BH, Shaw GM. bioRxiv : the preprint server for biology. 2025 Oct 6 -
Allergen-specific mRNA-lipid nanoparticle therapy for prevention and treatment of experimental allergy in mice.
Rochman Y, Kotliar M, Klingler AM, Rochman M, Alameh MG, Melamed JR, Osswald GA, Caldwell JM, Felton JM, Mack LE, Hargis J, Lewkowich IP, Barski A, Weissman D, Rothenberg ME. The Journal of clinical investigation. 2025 Nov 3;135(21) Epub 2025 Sep 23 -
Harnessing mRNA-lipid nanoparticles as innovative therapies for autoimmune diseases.
Razavi R, Kegel M, Muscat-Rivera J, Weissman D, Melamed JR. Molecular therapy. Methods & clinical development. 2025 Sep 11;33(3):101566 Epub 2025 Aug 18 -
Immunogenicity and safety of 'Comvigen', a bivalent SARS-CoV-2 vaccine, in comparison to Comirnaty bivalent vaccine in Thailand: a phase 2, non-inferiority randomised trial.
Jantarabenjakul W, Nantanee R, Puthanakit T, Gatechompol S, Avihingsanon A, Punrin S, Tantawichien T, Nitayaphan S, Thitithanyanont A, Buranapraditkun S, Jongkaewwattana A, Ketloy C, Prompetchara E, Lawpoolsri S, Wijagkanalan W, Alameh MG, Hong L, Samija M, Weissman D, Ruxrungtham K, ChulaVac006 Study Team. The Lancet regional health. Southeast Asia. 2025 Sep;40:100650 Epub 2025 Aug 15 -
Homologous and Heterologous Vaccination Regimens with mRNA and rVSV Platforms Induce Potent Immune Responses Against SFTSV Glycoprotein.
Manzoni TB, Westover JB, Lundgreen KA, Hicks PD, Petch RJ, Ort JT, Weissman D, Fan SHY, Hensley SE, Pardi N, Gowen BB, Bates P. Viruses. 2025 Aug 8;17(8) -
mRNA-LNP vaccine encoding the Plasmodium vivax circumsporozoite protein is highly immunogenic and confers protection in mice.
Limsalakpetch A, Kum-Arb U, Yongvanitchit K, Im-Erbsin R, Ubalee R, Waters N, Vesely BA, Muramatsu H, Weissman D, Tam YK, Yoshida S, Adams J, Yadava A, Pardi N, Pichyangkul S. Molecular therapy. Nucleic acids. 2025 Sep 9;36(3):102645 Epub 2025 Jul 30 -
Systemic delivery of biotherapeutic RNA to the myocardium transiently modulates cardiac contractility in vivo.
Shuvaev VV, Tam YK, Lee BW, Myerson JW, Herbst A, Kiseleva RY, Glassman PM, Parhiz H, Alameh MG, Pardi N, Muramatsu H, Shuvaeva TI, Arguiri E, Marcos-Contreras OA, Hood ED, Brysgel TV, Nong J, Papp TE, Eaton DM, Riley R, Palanki R, Musunuru K, Brenner JS, Mitchell MJ, Ferrari VA, Mui BL, Semple SC, Weppler SA, Atluri P, Margulies KB, Weissman D, Muzykantov VR. Proceedings of the National Academy of Sciences of the United States of America. 2025 Jul 22;122(29):e2409266122 Epub 2025 Jul 16 -
IL-12 mRNA-LNP promotes dermal resident memory CD4(+) T cell development.
Zabala-Peñafiel A, Gonzalez-Lombana C, Alameh MG, Sacramento LA, Mou Z, Phan AT, Aunins EA, Tam YK, Uzonna JE, Weissman D, Hunter CA, Scott P. NPJ vaccines. 2025 Jul 16;10(1):154 -
CD47 peptide-cloaked lipid nanoparticles promote cell-specific mRNA delivery.
Papp TE, Zeng J, Shahnawaz H, Akyianu A, Breda L, Yadegari A, Steward J, Shi R, Li Q, Mui BL, Tam YK, Weissman D, Rivella S, Shuvaev V, Muzykantov VR, Parhiz H. Molecular therapy : the journal of the American Society of Gene Therapy. 2025 Jul 2;33(7):3195-3208 Epub 2025 Mar 13 -
Harnessing the Electron-Withdrawing Inductive Effect of One-Component Ionizable Amphiphilic Janus Dendrimers Unveils Cation-π Interactions and Their Important Roles to Targeted mRNA Delivery.
Arshad M, Atochina-Vasserman EN, Maurya DS, Lu J, Ona N, Vasserman JA, McWilliams BC, Ni H, Berkihiser S, Park WJ, Weissman D, Percec V. Journal of the American Chemical Society. 2025 Jun 25;147(25):21347-21356 Epub 2025 Jun 13 -
Nonstabilized SARS-CoV-2 spike mRNA vaccination induces broadly neutralizing antibodies in nonhuman primates.
Malewana RD, Stalls V, May A, Lu X, Martinez DR, Schäfer A, Li D, Barr M, Sutherland LL, Lee E, Parks R, Beck WE, Newman A, Bock KW, Minai M, Nagata BM, DeMarco CT, Denny TN, Oguin TH 3rd, Rountree W, Wang Y, Mansouri K, Edwards RJ, Smith L, Sempowski GD, Eaton A, Muramatsu H, Henderson R, Tam Y, Barbosa C, Tang J, Cain DW, Santra S, Moore IN, Andersen H, Lewis MG, Golding H, Seder R, Khurana S, Montefiori DC, Pardi N, Weissman D, Baric RS, Acharya P, Haynes BF, Saunders KO. Science translational medicine. 2025 Jun 11;17(802):eadn5651 -
An Il12 mRNA-LNP adjuvant enhances mRNA vaccine-induced CD8 T cell responses.
Aunins EA, Phan AT, Alameh MG, Dwivedi G, Cruz-Morales E, Christian DA, Tam Y, Bunkofske ME, Peñafiel AZ, O'Dea KM, Merolle M, Furey C, Scott P, Vonderheide RH, Hensley SE, Kedl RM, Weissman D, Hunter CA. Science immunology. 2025 Jun 6;10(108):eads1328 -
SRSF3 knockdown-induced cellular senescence as a possible therapeutic strategy for non-small cell lung cancer.
Nakamichi S, von Muhlinen N, Yamada L, Melamed JR, Papp TE, Parhiz H, Weissman D, Horikawa I, Harris CC. bioRxiv : the preprint server for biology. 2025 May 9 -
BMP-2 mRNA-transfected BMSCs promote superior calvarial bone regeneration.
Surisaeng T, Wisitrasameewong W, Champaiboon C, Sa-Ard-Iam N, Chanamuangkon T, Thongnuek P, Tam YK, Muramatsu H, Weissman D, Pardi N, Pichyangkul S, Mahanonda R. Scientific reports. 2025 Apr 29;15(1):15022 -
Tick feeding or vaccination with tick antigens elicits immunity to the Ixodes scapularis exoproteome in guinea pigs and humans.
Hart TM, Cui Y, Telford SR, Marín-López A, Calloway K, Dai Y, Matias J, DePonte K, Jaycox J, DeBlasio M, Hoornstra D, Belperron AA, Cibichakravarthy B, Johnson EE, Alameh MG, Dwivedi G, Hovius JWR, Bockenstedt LK, Weissman D, Ring AM, Fikrig E. Science translational medicine. 2025 Mar 26;17(791):eads9207 -
Monitoring mRNA vaccine antigen expression in vivo using PET/CT.
Blizard GS, Dwivedi G, Pan YG, Hou C, Etersque JM, Said H, Chevrier A, Lavertu M, Ni H, Davis B, Tam Y, Cao Q, Mach RH, Weissman D, Alameh MG, Sellmyer MA. Nature communications. 2025 Mar 6;16(1):2234 -
Plug-and-play assembly of biodegradable ionizable lipids for potent mRNA delivery and gene editing in vivo.
Han X, Xu Y, Ricciardi A, Xu J, Palanki R, Chowdhary V, Xue L, Gong N, Alameh MG, Peranteau WH, Wilson JM, Weissman D, Mitchell MJ. bioRxiv : the preprint server for biology. 2025 Mar 1 -
Author Correction: Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals.
Connors J, Joyner D, Mege NJ, Cusimano GM, Bell MR, Marcy J, Taramangalam B, Kim KM, Lin PJC, Tam YK, Weissman D, Kutzler MA, Alameh MG, Haddad EK. Communications biology. 2025 Feb 22;8(1):285 -
Targeted delivery of TGF-β mRNA to murine lung parenchyma using one-component ionizable amphiphilic Janus Dendrimers.
Meshanni JA, Stevenson ER, Zhang D, Sun R, Ona NA, Reagan EK, Abramova E, Guo CJ, Wilkinson M, Baboo I, Yang Y, Pan L, Maurya DS, Percec V, Li Y, Gow A, Weissman D, Atochina-Vasserman EN. Nature communications. 2025 Feb 21;16(1):1806 -
iDC-targeting PfCSP mRNA vaccine confers superior protection against Plasmodium compared to conventional mRNA.
Yanik S, Venkatesh V, Gordy JT, Alameh MG, Meza J, Li Y, Glass E, Flores-Garcia Y, Tam Y, Chaiyawong N, Sarkar D, Weissman D, Markham R, Srinivasan P. NPJ vaccines. 2025 Feb 19;10(1):34 -
Publisher Correction: Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia.
Swingle KL, Hamilton AG, Safford HC, Geisler HC, Thatte AS, Palanki R, Murray AM, Han EL, Mukalel AJ, Han X, Joseph RA, Ghalsasi AA, Alameh MG, Weissman D, Mitchell MJ. Nature. 2025 Feb;638(8051):E33 -
Multiarm-Assisted Design of Dendron-like Degradable Ionizable Lipids Facilitates Systemic mRNA Delivery to the Spleen.
Xue L, Xiong X, Zhao G, Molina-Arocho W, Palanki R, Xiao Z, Han X, Yoon IC, Figueroa-Espada CG, Xu J, Gong N, Shi Q, Chen Q, Alameh MG, Vaughan AE, Haldar M, Wang K, Weissman D, Mitchell MJ. Journal of the American Chemical Society. 2025 Jan 15;147(2):1542-1552 Epub 2025 Jan 1 -
Toward a Complete Elucidation of the Primary Structure-Activity in Pentaerythritol-Based One-Component Ionizable Amphiphilic Janus Dendrimers for In Vivo Delivery of Luc-mRNA.
Sahoo D, Atochina-Vasserman EN, Lu J, Maurya DS, Ona N, Vasserman JA, Ni H, Berkihiser S, Park WJ, Weissman D, Percec V. Biomacromolecules. 2025 Jan 13;26(1):726-737 Epub 2024 Dec 17 -
Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery.
Xue L, Zhao G, Gong N, Han X, Shepherd SJ, Xiong X, Xiao Z, Palanki R, Xu J, Swingle KL, Warzecha CC, El-Mayta R, Chowdhary V, Yoon IC, Xu J, Cui J, Shi Y, Alameh MG, Wang K, Wang L, Pochan DJ, Weissman D, Vaughan AE, Wilson JM, Mitchell MJ. Nature nanotechnology. 2025 Jan;20(1):132-143 Epub 2024 Oct 1 -
Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia.
Swingle KL, Hamilton AG, Safford HC, Geisler HC, Thatte AS, Palanki R, Murray AM, Han EL, Mukalel AJ, Han X, Joseph RA, Ghalsasi AA, Alameh MG, Weissman D, Mitchell MJ. Nature. 2025 Jan;637(8045):412-421 Epub 2024 Dec 11 -
Restoring hematopoietic stem and progenitor cell function in Fancc (-/-) mice by in situ delivery of RNA lipid nanoparticles.
Banda O, Adams SE, Omer L, Jung SK, Said H, Phoka T, Tam Y, Weissman D, Rivella S, Alameh MG, Kurre P. Molecular therapy. Nucleic acids. 2025 Mar 11;36(1):102423 Epub 2024 Dec 12 -
Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery.
Gong N, Zhong W, Alameh MG, Han X, Xue L, El-Mayta R, Zhao G, Vaughan AE, Qin Z, Xu F, Hamilton AG, Kim D, Xu J, Kim J, Teng X, Li J, Liang XJ, Weissman D, Guo W, Mitchell MJ. Nature materials. 2024 Dec;23(12):1736-1747 Epub 2024 Sep 2 -
CD8(+) T cells exacerbate AD-like symptoms in mouse model of amyloidosis.
Wang X, Campbell B, Bodogai M, McDevitt RA, Patrikeev A, Gusev F, Ragonnaud E, Kumaraswami K, Shirenova S, Vardy K, Alameh MG, Weissman D, Ishikawa-Ankerhold H, Okun E, Rogaev E, Biragyn A. Brain, behavior, and immunity. 2024 Nov;122:444-455 Epub 2024 Aug 25 -
Thin-film freeze-drying of an influenza virus hemagglutinin mRNA vaccine in unilamellar lipid nanoparticles with blebs.
Li Q, Shi R, Xu H, AboulFotouh K, Sung MMH, Oguin TH, Hayes M, Moon C, Dao HM, Ni H, Sahakijpijarn S, Cano C, Davenport GJ, Williams RO 3rd, Le Huray J, Cui Z, Weissman D. Journal of controlled release : official journal of the Controlled Release Society. 2024 Nov;375:829-838 Epub 2024 Oct 10 -
Optimization of the activity and biodegradability of ionizable lipids for mRNA delivery via directed chemical evolution.
Han X, Alameh MG, Xu Y, Palanki R, El-Mayta R, Dwivedi G, Swingle KL, Xu J, Gong N, Xue L, Shi Q, Yoon IC, Warzecha CC, Wilson JM, Weissman D, Mitchell MJ. Nature biomedical engineering. 2024 Nov;8(11):1412-1424 Epub 2024 Nov 22 -
Salp14 epitope-based mRNA vaccination induces early recognition of a tick bite.
Cui Y, Cibichakravarthy B, Tang X, Alameh MG, Dwivedi G, Weissman D, Fikrig E. Vaccine. 2024 Oct 24;42(24):126304 Epub 2024 Sep 5 -
Accelerated Ten-Gram-Scale Synthesis of One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer 97.
Arshad M, Atochina-Vasserman EN, Chenna SS, Maurya DS, Shalihin MI, Sahoo D, Lewis AC, Lewis JJ, Ona N, Vasserman JA, Ni H, Park WJ, Weissman D, Percec V. Biomacromolecules. 2024 Oct 14;25(10):6871-6882 Epub 2024 Oct 3 -
Use of HSC-targeted LNP to generate a mouse model of lethal α-thalassemia and treatment via lentiviral gene therapy.
Chappell ME, Breda L, Tricoli L, Guerra A, Jarocha D, Castruccio Castracani C, Papp TE, Tanaka N, Hamilton N, Triebwasser MP, Ghiaccio V, Fedorky MT, Gollomp KL, Bochenek V, Roche AM, Everett JK, Cook EJ, Bushman FD, Teawtrakul N, Glentis S, Kattamis A, Mui BL, Tam YK, Weissman D, Abdulmalik O, Parhiz H, Rivella S. Blood. 2024 Oct 10;144(15):1633-1645 -
A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection.
Alameh MG, Semon A, Bayard NU, Pan YG, Dwivedi G, Knox J, Glover RC, Rangel PC, Tanes C, Bittinger K, She Q, Hu H, Bonam SR, Maslanka JR, Planet PJ, Moustafa AM, Davis B, Chevrier A, Beattie M, Ni H, Blizard G, Furth EE, Mach RH, Lavertu M, Sellmyer MA, Tam Y, Abt MC, Weissman D, Zackular JP. Science (New York, N.Y.). 2024 Oct 4;386(6717):69-75 Epub 2024 Oct 3 -
Orthogonal Design of Experiments for Engineering of Lipid Nanoparticles for mRNA Delivery to the Placenta.
Safford HC, Swingle KL, Geisler HC, Hamilton AG, Thatte AS, Ghalsasi AA, Billingsley MM, Alameh MG, Weissman D, Mitchell MJ. Small (Weinheim an der Bergstrasse, Germany). 2024 Oct;20(41):e2303568 Epub 2023 Aug 3 -
Fast and facile synthesis of amidine-incorporated degradable lipids for versatile mRNA delivery in vivo.
Han X, Alameh MG, Gong N, Xue L, Ghattas M, Bojja G, Xu J, Zhao G, Warzecha CC, Padilla MS, El-Mayta R, Dwivedi G, Xu Y, Vaughan AE, Wilson JM, Weissman D, Mitchell MJ. Nature chemistry. 2024 Oct;16(10):1687-1697 Epub 2024 Jul 9 -
Bivalent norovirus mRNA vaccine elicits cellular and humoral responses protecting human enteroids from GII.4 infection.
Atochina-Vasserman EN, Lindesmith LC, Mirabelli C, Ona NA, Reagan EK, Brewer-Jensen PD, Mercado-Lopez X, Shahnawaz H, Meshanni JA, Baboo I, Mallory ML, Zweigart MR, May SR, Mui BL, Tam YK, Wobus CE, Baric RS, Weissman D. NPJ vaccines. 2024 Oct 1;9(1):182 -
Comparison of the immunogenicity of mRNA-encoded and protein HIV-1 Env-ferritin nanoparticle designs.
Mu Z, Whitley J, Martik D, Sutherland L, Newman A, Barr M, Parks R, Wiehe K, Cain DW, Hodges KZ, Venkatayogi S, Lee EM, Smith L, Mansouri K, Edwards RJ, Wang Y, Rountree W, Alameh M-G, Tam Y, Barbosa C, Tomai M, Lewis MG, Santrai S, Maughan M, Tian M, Alt FW, Weissman D, Saunders KO, Haynes BF. Journal of virology. 2024 Sep 17;98(9):e0013724 Epub 2024 Aug 13 -
Broad protection and respiratory immunity of dual mRNA vaccination against SARS-CoV-2 variants.
Hajnik RL, Plante JA, Reddy Bonam S, Rafael GH, Liang Y, Hazell NC, Walker J, Reyna RA, Walker DH, Alameh MG, Weissman D, Weaver SC, Plante KS, Hu H. NPJ vaccines. 2024 Sep 4;9(1):160 -
In utero delivery of targeted ionizable lipid nanoparticles facilitates in vivo gene editing of hematopoietic stem cells.
Palanki R, Riley JS, Bose SK, Luks V, Dave A, Kus N, White BM, Ricciardi AS, Swingle KL, Xue L, Sung D, Thatte AS, Safford HC, Chaluvadi VS, Carpenter M, Han EL, Maganti R, Hamilton AG, Mrksich K, Billingsley MB, Zoltick PW, Alameh MG, Weissman D, Mitchell MJ, Peranteau WH. Proceedings of the National Academy of Sciences of the United States of America. 2024 Aug 6;121(32):e2400783121 Epub 2024 Jul 30 -
Enhancing in situ cancer vaccines using delivery technologies.
Gong N, Alameh MG, El-Mayta R, Xue L, Weissman D, Mitchell MJ. Nature reviews. Drug discovery. 2024 Aug;23(8):607-625 Epub 2024 Jul 1 -
mRNA lipid nanoparticles expressing cell-surface cleavage independent HIV Env trimers elicit autologous tier-2 neutralizing antibodies.
Guenaga J, Alirezaei M, Feng Y, Alameh MG, Lee WH, Baboo S, Cluff J, Wilson R, Bale S, Ozorowski G, Lin P, Tam Y, Diedrich JK, Yates JR 3rd, Paulson JC, Ward AB, Weissman D, Wyatt RT. Frontiers in immunology. 2024;15:1426232 Epub 2024 Jul 25 -
Targeted delivery of TGF-β mRNA to lung parenchyma using one-component ionizable amphiphilic Janus Dendrimers.
Atochina-Vasserman E, Meshanni J, Stevenson E, Zhang D, Sun R, Ona N, Reagan E, Abramova E, Guo CJ, Wilkinson M, Baboo I, Yang Y, Pan L, Maurya D, Percec V, Li Y, Gow A, Weissman D. Research square. 2024 Jul 12 -
Immature dendritic cell-targeting mRNA vaccine expressing PfCSP enhances protective immune responses against Plasmodium liver infection.
Yanik S, Venkatesh V, Gordy JT, Gabriel-Alameh M, Meza J, Li Y, Glass E, Flores-Garcia Y, Tam Y, Chaiyawong N, Sarkar D, Weissman D, Markham R, Srinivasan P. Research square. 2024 Jul 9 -
Oxidized mRNA Lipid Nanoparticles for In Situ Chimeric Antigen Receptor Monocyte Engineering.
Mukalel AJ, Hamilton AG, Billingsley MM, Li J, Thatte AS, Han X, Safford HC, Padilla MS, Papp T, Parhiz H, Weissman D, Mitchell MJ. Advanced functional materials. 2024 Jul 3;34(27) Epub 2024 Mar 5 -
Evaluation of mRNA-LNP and adjuvanted protein SARS-CoV-2 vaccines in a maternal antibody mouse model.
England RN, Drapeau EM, Alameh MG, Hosseinzadeh R, Weissman D, Hensley SE. NPJ vaccines. 2024 Jun 18;9(1):110 -
Transient growth factor expression via mRNA in lipid nanoparticles promotes hepatocyte cell therapy in mice.
Smith AR, Rizvi F, Everton E, Adeagbo A, Wu S, Tam Y, Muramatsu H, Pardi N, Weissman D, Gouon-Evans V. Nature communications. 2024 Jun 12;15(1):5010 -
Physicochemical Targeting of Lipid Nanoparticles to the Lungs Induces Clotting: Mechanisms and Solutions.
Omo-Lamai S, Zamora ME, Patel MN, Wu J, Nong J, Wang Z, Peshkova A, Majumder A, Melamed JR, Chase LS, Essien EO, Weissman D, Muzykantov VR, Marcos-Contreras OA, Myerson JW, Brenner JS. Advanced materials (Deerfield Beach, Fla.). 2024 Jun;36(26):e2312026 Epub 2024 Mar 13 -
Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy.
Metzloff AE, Padilla MS, Gong N, Billingsley MM, Han X, Merolle M, Mai D, Figueroa-Espada CG, Thatte AS, Haley RM, Mukalel AJ, Hamilton AG, Alameh MG, Weissman D, Sheppard NC, June CH, Mitchell MJ. Advanced materials (Deerfield Beach, Fla.). 2024 Jun;36(26):e2313226 Epub 2024 Mar 15 -
Immunization with PfGBP130 generates antibodies that inhibit RBC invasion by P. falciparum parasites.
Johnson Y, Shakri AR, Pond-Tor S, Jnawali A, Najrana T, Wu H, Badhai J, Alameh MG, Weissman D, Kabyemela E, Duffy P, Fried M, Kurtis J, Raj DK. Frontiers in immunology. 2024;15:1350560 Epub 2024 May 28 -
Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus.
Furey C, Scher G, Ye N, Kercher L, DeBeauchamp J, Crumpton JC, Jeevan T, Patton C, Franks J, Rubrum A, Alameh MG, Fan SHY, Phan AT, Hunter CA, Webby RJ, Weissman D, Hensley SE. Nature communications. 2024 May 23;15(1):4350 -
Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice.
Bonam SR, Hazell NC, Mathew MJ, Liang Y, Zhang X, Wei Z, Alameh MG, Weissman D, Hu H. Vaccines. 2024 May 15;12(5) -
Mutation-guided vaccine design: A process for developing boosting immunogens for HIV broadly neutralizing antibody induction.
Wiehe K, Saunders KO, Stalls V, Cain DW, Venkatayogi S, Martin Beem JS, Berry M, Evangelous T, Henderson R, Hora B, Xia SM, Jiang C, Newman A, Bowman C, Lu X, Bryan ME, Bal J, Sanzone A, Chen H, Eaton A, Tomai MA, Fox CB, Tam YK, Barbosa C, Bonsignori M, Muramatsu H, Alam SM, Montefiori DC, Williams WB, Pardi N, Tian M, Weissman D, Alt FW, Acharya P, Haynes BF. Cell host & microbe. 2024 May 8;32(5):693-709.e7 Epub 2024 Apr 25 -
Targeting lipid nanoparticles to the blood-brain barrier to ameliorate acute ischemic stroke.
Nong J, Glassman PM, Shuvaev VV, Reyes-Esteves S, Descamps HC, Kiseleva RY, Papp TE, Alameh MG, Tam YK, Mui BL, Omo-Lamai S, Zamora ME, Shuvaeva T, Arguiri E, Gong X, Brysgel TV, Tan AW, Woolfork AG, Weljie A, Thaiss CA, Myerson JW, Weissman D, Kasner SE, Parhiz H, Muzykantov VR, Brenner JS, Marcos-Contreras OA. Molecular therapy : the journal of the American Society of Gene Therapy. 2024 May 1;32(5):1344-1358 Epub 2024 Mar 7 -
Evaluation of the immunogenicity of an mRNA vectored Nipah virus vaccine candidate in pigs.
Pedrera M, McLean RK, Medfai L, Thakur N, Todd S, Marsh G, Bailey D, Donofrio G, Muramatsu H, Pardi N, Weissman D, Graham SP. Frontiers in immunology. 2024;15:1384417 Epub 2024 Apr 25 -
Emerging strategies for nanomedicine in autoimmunity.
Thatte AS, Billingsley MM, Weissman D, Melamed JR, Mitchell MJ. Advanced drug delivery reviews. 2024 Apr;207:115194 Epub 2024 Feb 10 -
IL7 increases targeted lipid nanoparticle-mediated mRNA expression in T cells in vitro and in vivo by enhancing T cell protein translation.
Tilsed CM, Sadiq BA, Papp TE, Areesawangkit P, Kimura K, Noguera-Ortega E, Scholler J, Cerda N, Aghajanian H, Bot A, Mui B, Tam Y, Weissman D, June CH, Albelda SM, Parhiz H. Proceedings of the National Academy of Sciences of the United States of America. 2024 Mar 26;121(13):e2319856121 Epub 2024 Mar 21 -
mRNA-based therapeutics: looking beyond COVID-19 vaccines.
Parhiz H, Atochina-Vasserman EN, Weissman D. Lancet (London, England). 2024 Mar 23;403(10432):1192-1204 Epub 2024 Mar 7 -
Physiologically based modeling of LNP-mediated delivery of mRNA in the vascular system.
Parhiz H, Shuvaev VV, Li Q, Papp TE, Akyianu AA, Shi R, Yadegari A, Shahnawaz H, Semple SC, Mui BL, Weissman D, Muzykantov VR, Glassman PM. Molecular therapy. Nucleic acids. 2024 Jun 11;35(2):102175 Epub 2024 Mar 18 -
In Vivo mRNA CAR T Cell Engineering via Targeted Ionizable Lipid Nanoparticles with Extrahepatic Tropism.
Billingsley MM, Gong N, Mukalel AJ, Thatte AS, El-Mayta R, Patel SK, Metzloff AE, Swingle KL, Han X, Xue L, Hamilton AG, Safford HC, Alameh MG, Papp TE, Parhiz H, Weissman D, Mitchell MJ. Small (Weinheim an der Bergstrasse, Germany). 2024 Mar;20(11):e2304378 Epub 2023 Dec 10 -
High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models.
Xue L, Hamilton AG, Zhao G, Xiao Z, El-Mayta R, Han X, Gong N, Xiong X, Xu J, Figueroa-Espada CG, Shepherd SJ, Mukalel AJ, Alameh MG, Cui J, Wang K, Vaughan AE, Weissman D, Mitchell MJ. Nature communications. 2024 Feb 29;15(1):1884 -
In situ combinatorial synthesis of degradable branched lipidoids for systemic delivery of mRNA therapeutics and gene editors.
Han X, Xu J, Xu Y, Alameh MG, Xue L, Gong N, El-Mayta R, Palanki R, Warzecha CC, Zhao G, Vaughan AE, Wilson JM, Weissman D, Mitchell MJ. Nature communications. 2024 Feb 26;15(1):1762 -
Multivalent cytomegalovirus glycoprotein B nucleoside modified mRNA vaccines did not demonstrate a greater antibody breadth.
Wang HY, Li L, Nelson CS, Barfield R, Valencia S, Chan C, Muramatsu H, Lin PJC, Pardi N, An Z, Weissman D, Permar SR. NPJ vaccines. 2024 Feb 20;9(1):38 -
The Constitutional Isomerism of One-Component Ionizable Amphiphilic Janus Dendrimers Orchestrates the Total and Targeted Activities of mRNA Delivery.
Sahoo D, Atochina-Vasserman EN, Maurya DS, Arshad M, Chenna SS, Ona N, Vasserman JA, Ni H, Weissman D, Percec V. Journal of the American Chemical Society. 2024 Feb 14;146(6):3627-3634 Epub 2024 Feb 2 -
TGF-βR2 signaling coordinates pulmonary vascular repair after viral injury in mice and human tissue.
Zhao G, Xue L, Weiner AI, Gong N, Adams-Tzivelekidis S, Wong J, Gentile ME, Nottingham AN, Basil MC, Lin SM, Niethamer TK, Diamond JM, Bermudez CA, Cantu E, Han X, Cao Y, Alameh MG, Weissman D, Morrisey EE, Mitchell MJ, Vaughan AE. Science translational medicine. 2024 Jan 31;16(732):eadg6229 -
Phase II prefusion non-stabilised Covid-19 mRNA vaccine randomised study.
Puthanakit T, Prompetchara E, Gatechompol S, Ketloy C, Thitithanyanont A, Jongkaewwattana A, Buranapraditkun S, Ubolyam S, Kerr SJ, Sophonphan J, Apornpong T, Kittanamongkolchai W, Siwamogsatham S, Sriplienchan S, Patarakul K, Theerawit T, Promsena P, Nantanee R, Manomaisantiphap S, Chokyakorn S, Hong L, Samija M, Montefiori DC, Gao H, Eaton A, Wijagkanalan W, Alameh MG, Weissman D, Ruxrungtham K, ChulaVac001-Phase 2 study team. Scientific reports. 2024 Jan 29;14(1):2373 -
Hepatitis C virus modified sE2(F442NYT) as an antigen in candidate vaccine facilitates human immune cell activation.
Haga Y, Meyer K, Sung MMH, Reagan EK, Weissman D, Ray R. Journal of virology. 2024 Jan 23;98(1):e0180923 Epub 2023 Dec 12 -
mRNA vaccination of rabbits alters the fecundity, but not the attachment, of adult Ixodes scapularis.
Matias J, Cui Y, Lynn GE, DePonte K, Mesquita E, Muramatsu H, Alameh MG, Dwivedi G, Tam YK, Pardi N, Weissman D, Fikrig E. Scientific reports. 2024 Jan 4;14(1):496 -
Vaccine induction of CD4-mimicking HIV-1 broadly neutralizing antibody precursors in macaques.
Saunders KO, Counts J, Thakur B, Stalls V, Edwards R, Manne K, Lu X, Mansouri K, Chen Y, Parks R, Barr M, Sutherland L, Bal J, Havill N, Chen H, Machiele E, Jamieson N, Hora B, Kopp M, Janowska K, Anasti K, Jiang C, Van Itallie E, Venkatayogi S, Eaton A, Henderson R, Barbosa C, Alam SM, Santra S, Weissman D, Moody MA, Cain DW, Tam YK, Lewis M, Williams WB, Wiehe K, Montefiori DC, Acharya P, Haynes BF. Cell. 2024 Jan 4;187(1):79-94.e24 -
Systematic development of ionizable lipid nanoparticles for placental mRNA delivery using a design of experiments approach.
Young RE, Nelson KM, Hofbauer SI, Vijayakumar T, Alameh MG, Weissman D, Papachristou C, Gleghorn JP, Riley RS. Bioactive materials. 2024 Apr;34:125-137 Epub 2023 Dec 22 -
Broadly neutralizing antibody induction by non-stabilized SARS-CoV-2 Spike mRNA vaccination in nonhuman primates.
Malewana RD, Stalls V, May A, Lu X, Martinez DR, Schäfer A, Li D, Barr M, Sutherland LL, Lee E, Parks R, Beck WE, Newman A, Bock KW, Minai M, Nagata BM, DeMarco CT, Denny TN, Oguin TH 3rd, Rountree W, Wang Y, Mansouri K, Edwards RJ, Sempowski GD, Eaton A, Muramatsu H, Henderson R, Tam Y, Barbosa C, Tang J, Cain DW, Santra S, Moore IN, Andersen H, Lewis MG, Golding H, Seder R, Khurana S, Montefiori DC, Pardi N, Weissman D, Baric RS, Acharya P, Haynes BF, Saunders KO. bioRxiv : the preprint server for biology. 2023 Dec 19 -
VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis.
Rizvi F, Lee YR, Diaz-Aragon R, Bawa PS, So J, Florentino RM, Wu S, Sarjoo A, Truong E, Smith AR, Wang F, Everton E, Ostrowska A, Jung K, Tam Y, Muramatsu H, Pardi N, Weissman D, Soto-Gutierrez A, Shin D, Gouon-Evans V. Cell stem cell. 2023 Dec 7;30(12):1640-1657.e8 Epub 2023 Nov 28 -
Ionizable Lipid Nanoparticles with Integrated Immune Checkpoint Inhibition for mRNA CAR T Cell Engineering.
Hamilton AG, Swingle KL, Joseph RA, Mai D, Gong N, Billingsley MM, Alameh MG, Weissman D, Sheppard NC, June CH, Mitchell MJ. Advanced healthcare materials. 2023 Dec;12(30):e2301515 Epub 2023 Aug 31 -
The Regulation of Nucleic Acid Vaccine Responses by the Microbiome.
Johnson AMF, Hager K, Alameh MG, Van P, Potchen N, Mayer-Blackwell K, Fiore-Gartland A, Minot S, Lin PJC, Tam YK, Weissman D, Kublin JG. Journal of immunology (Baltimore, Md. : 1950). 2023 Dec 1;211(11):1680-1692 -
Progress with induction of HIV broadly neutralizing antibodies in the Duke Consortia for HIV/AIDS Vaccine Development.
Haynes BF, Wiehe K, Alam SM, Weissman D, Saunders KO. Current opinion in HIV and AIDS. 2023 Nov 1;18(6):300-308 Epub 2023 Sep 25 -
Development of an mRNA-lipid nanoparticle vaccine against Lyme disease.
Pine M, Arora G, Hart TM, Bettini E, Gaudette BT, Muramatsu H, Tombácz I, Kambayashi T, Tam YK, Brisson D, Allman D, Locci M, Weissman D, Fikrig E, Pardi N. Molecular therapy : the journal of the American Society of Gene Therapy. 2023 Sep 6;31(9):2702-2714 Epub 2023 Aug 2 -
Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines.
Han X, Alameh MG, Butowska K, Knox JJ, Lundgreen K, Ghattas M, Gong N, Xue L, Xu Y, Lavertu M, Bates P, Xu J, Nie G, Zhong Y, Weissman D, Mitchell MJ. Nature nanotechnology. 2023 Sep;18(9):1105-1114 Epub 2023 Jun 26 -
Targeted and Equally Distributed Delivery of mRNA to Organs with Pentaerythritol-Based One-Component Ionizable Amphiphilic Janus Dendrimers.
Lu J, Atochina-Vasserman EN, Maurya DS, Sahoo D, Ona N, Reagan EK, Ni H, Weissman D, Percec V. Journal of the American Chemical Society. 2023 Aug 30;145(34):18760-18766 Epub 2023 Aug 22 -
Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines.
Shepherd SJ, Han X, Mukalel AJ, El-Mayta R, Thatte AS, Wu J, Padilla MS, Alameh MG, Srikumar N, Lee D, Weissman D, Issadore D, Mitchell MJ. Proceedings of the National Academy of Sciences of the United States of America. 2023 Aug 15;120(33):e2303567120 Epub 2023 Aug 9 -
Author Correction: mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum.
Hayashi CTH, Cao Y, Clark LC, Tripathi AK, Zavala F, Dwivedi G, Knox J, Alameh MG, Lin PJC, Tam YK, Weissman D, Kumar N. NPJ vaccines. 2023 Aug 11;8(1):115 -
Specific mRNA lipid nanoparticles and acquired resistance to ticks.
Matias J, Cui Y, Tang X, Sajid A, Arora G, Wu MJ, DePonte K, Muramatsu H, Tam YK, Narasimhan S, Pardi N, Weissman D, Fikrig E. Vaccine. 2023 Jul 31;41(34):4996-5002 Epub 2023 Jul 6 -
In vivo hematopoietic stem cell modification by mRNA delivery.
Breda L, Papp TE, Triebwasser MP, Yadegari A, Fedorky MT, Tanaka N, Abdulmalik O, Pavani G, Wang Y, Grupp SA, Chou ST, Ni H, Mui BL, Tam YK, Weissman D, Rivella S, Parhiz H. Science (New York, N.Y.). 2023 Jul 28;381(6656):436-443 Epub 2023 Jul 27 -
Targeted Nanocarriers Co-Opting Pulmonary Intravascular Leukocytes for Drug Delivery to the Injured Brain.
Nong J, Glassman PM, Myerson JW, Zuluaga-Ramirez V, Rodriguez-Garcia A, Mukalel A, Omo-Lamai S, Walsh LR, Zamora ME, Gong X, Wang Z, Bhamidipati K, Kiseleva RY, Villa CH, Greineder CF, Kasner SE, Weissman D, Mitchell MJ, Muro S, Persidsky Y, Brenner JS, Muzykantov VR, Marcos-Contreras OA. ACS nano. 2023 Jul 25;17(14):13121-13136 Epub 2023 Jul 11 -
Ionizable Lipid Nanoparticles for Therapeutic Base Editing of Congenital Brain Disease.
Palanki R, Bose SK, Dave A, White BM, Berkowitz C, Luks V, Yaqoob F, Han E, Swingle KL, Menon P, Hodgson E, Biswas A, Billingsley MM, Li L, Yiping F, Carpenter M, Trokhan A, Yeo J, Johana N, Wan TY, Alameh MG, Bennett FC, Storm PB, Jain R, Chan J, Weissman D, Mitchell MJ, Peranteau WH. ACS nano. 2023 Jul 25;17(14):13594-13610 Epub 2023 Jul 17 -
A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1.
Egan KP, Awasthi S, Tebaldi G, Hook LM, Naughton AM, Fowler BT, Beattie M, Alameh MG, Weissman D, Cohen GH, Friedman HM. Viruses. 2023 Jun 30;15(7) -
A mosquito AgTRIO mRNA vaccine contributes to immunity against malaria.
Chuang YM, Alameh MG, Abouneameh S, Raduwan H, Ledizet M, Weissman D, Fikrig E. NPJ vaccines. 2023 Jun 7;8(1):88 -
Screening Libraries to Discover Molecular Design Principles for the Targeted Delivery of mRNA with One-Component Ionizable Amphiphilic Janus Dendrimers Derived from Plant Phenolic Acids.
Lu J, Atochina-Vasserman EN, Maurya DS, Shalihin MI, Zhang D, Chenna SS, Adamson J, Liu M, Shah HUR, Shah H, Xiao Q, Queeley B, Ona NA, Reagan EK, Ni H, Sahoo D, Peterca M, Weissman D, Percec V. Pharmaceutics. 2023 May 23;15(6) -
Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus.
Furey C, Ye N, Kercher L, DeBeauchamp J, Crumpton JC, Jeevan T, Patton C, Franks J, Alameh MG, Fan SHY, Phan AT, Hunter CA, Webby RJ, Weissman D, Hensley SE. bioRxiv : the preprint server for biology. 2023 Apr 30 -
Immunogenicity and protective efficacy of SARS-CoV-2 mRNA vaccine encoding secreted non-stabilized spike in female mice.
Prompetchara E, Ketloy C, Alameh MG, Tharakhet K, Kaewpang P, Yostrerat N, Pitakpolrat P, Buranapraditkun S, Manopwisedjaroen S, Thitithanyanont A, Jongkaewwattana A, Hunsawong T, Im-Erbsin R, Reed M, Wijagkanalan W, Patarakul K, Techawiwattanaboon T, Palaga T, Lam K, Heyes J, Weissman D, Ruxrungtham K. Nature communications. 2023 Apr 21;14(1):2309 -
Breast tumors interfere with endothelial TRAIL at the premetastatic niche to promote cancer cell seeding.
Riera-Domingo C, Leite-Gomes E, Charatsidou I, Zhao P, Carrá G, Cappellesso F, Mourao L, De Schepper M, Liu D, Serneels J, Alameh MG, Shuvaev VV, Geukens T, Isnaldi E, Prenen H, Weissman D, Muzykantov VR, Soenen S, Desmedt C, Scheele CLGJ, Sablina A, Di Matteo M, Martín-Pérez R, Mazzone M. Science advances. 2023 Mar 22;9(12):eadd5028 -
Hepatitis C virus E1 and modified E2 delivered from an mRNA vaccine induces protective immunity.
Patra T, Meyer K, Haga Y, Reagan EK, Weissman D, Ray R. NPJ vaccines. 2023 Mar 18;8(1):42 -
Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy.
Swingle KL, Safford HC, Geisler HC, Hamilton AG, Thatte AS, Billingsley MM, Joseph RA, Mrksich K, Padilla MS, Ghalsasi AA, Alameh MG, Weissman D, Mitchell MJ. Journal of the American Chemical Society. 2023 Mar 1;145(8):4691-4706 Epub 2023 Feb 15 -
Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals.
Connors J, Joyner D, Mege NJ, Cusimano GM, Bell MR, Marcy J, Taramangalam B, Kim KM, Lin PJC, Tam YK, Weissman D, Kutzler MA, Alameh MG, Haddad EK. Communications biology. 2023 Feb 17;6(1):188 -
Nucleoside-Modified mRNA-Based Influenza Vaccines Circumvent Problems Associated with H3N2 Vaccine Strain Egg Adaptation.
Gouma S, Furey C, Santos JJS, Parkhouse K, Weirick M, Muramatsu H, Pardi N, Fan SHY, Weissman D, Hensley SE. Journal of virology. 2023 Jan 31;97(1):e0172322 Epub 2022 Dec 19 -
Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer.
Melamed JR, Yerneni SS, Arral ML, LoPresti ST, Chaudhary N, Sehrawat A, Muramatsu H, Alameh MG, Pardi N, Weissman D, Gittes GK, Whitehead KA. Science advances. 2023 Jan 27;9(4):eade1444 -
Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis.
Han X, Gong N, Xue L, Billingsley MM, El-Mayta R, Shepherd SJ, Alameh MG, Weissman D, Mitchell MJ. Nature communications. 2023 Jan 17;14(1):75 -
Nucleoside-modified mRNA vaccines yield robust blocking antibody responses against major house dust mite allergens.
Jitthamstaporn S, Inthong R, Audomsun D, Chanasit S, Thanasarnthungcharoen C, Lin PJC, Weissman D, Pardi N, Jacquet A. Allergy. 2023 Jan;78(1):315-318 Epub 2022 Sep 28 -
Restoration of Motor Function through Delayed Intraspinal Delivery of Human IL-10-Encoding Nucleoside-Modified mRNA after Spinal Cord Injury.
Gál L, Bellák T, Marton A, Fekécs Z, Weissman D, Török D, Biju R, Vizler C, Kristóf R, Beattie MB, Lin PJC, Pardi N, Nógrádi A, Pajer K. Research (Washington, D.C.). 2023;6:0056 Epub 2023 Mar 9 -
Exploring in vitro expression and immune potency in mice using mRNA encoding the Plasmodium falciparum malaria antigen, CelTOS.
Waghela IN, Mallory KL, Taylor JA, Schneider CG, Savransky T, Janse CJ, Lin PJC, Tam YK, Weissman D, Angov E. Frontiers in immunology. 2022;13:1026052 Epub 2022 Dec 15 -
SARS-CoV-2 mRNA-based vaccines in the Aicardi Goutières Syndrome.
Takanohashi A, Alameh MG, Woidill S, Hacker J, Davis B, Helman G, Gavazzi F, Adang L, D'Aiello R, Winters P, Cordova D, Khandaker T, Ni H, Tam Y, Lin P, Weissman D, Shults J, Vanderver A. Molecular genetics and metabolism. 2022 Dec;137(4):320-327 Epub 2022 Oct 10 -
Safety and immunogenicity of a prefusion non-stabilized spike protein mRNA COVID-19 vaccine: a phase I trial.
Gatechompol S, Kittanamongkolchai W, Ketloy C, Prompetchara E, Thitithanyanont A, Jongkaewwattana A, Buranapraditkun S, Alameh MG, Ubolyam S, Sophonphan J, Apornpong T, Kerr S, Kamarulzaman A, Siwamogsatham S, Kroon E, Puthanakit T, Patarakul K, Palaga T, Wijagkanalan W, Carpenter A, Hong L, Weissman D, Ruxrungtham K, ChulaVAC-001 study team. Nature microbiology. 2022 Dec;7(12):1987-1995 Epub 2022 Nov 14 -
mRNA-LNP expressing PfCSP and Pfs25 vaccine candidates targeting infection and transmission of Plasmodium falciparum.
Hayashi CTH, Cao Y, Clark LC, Tripathi AK, Zavala F, Dwivedi G, Knox J, Alameh MG, Lin PJC, Tam YK, Weissman D, Kumar N. NPJ vaccines. 2022 Dec 1;7(1):155 -
A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes.
Arevalo CP, Bolton MJ, Le Sage V, Ye N, Furey C, Muramatsu H, Alameh MG, Pardi N, Drapeau EM, Parkhouse K, Garretson T, Morris JS, Moncla LH, Tam YK, Fan SHY, Lakdawala SS, Weissman D, Hensley SE. Science (New York, N.Y.). 2022 Nov 25;378(6622):899-904 Epub 2022 Nov 24 -
Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity.
Liu W, Alameh MG, Yang JF, Xu JR, Lin PJC, Tam YK, Weissman D, You J. International journal of molecular sciences. 2022 Nov 22;23(23) -
Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals.
Connors J, Joyner D, Mege N, Cusimano G, Bell M, Marcy J, Taramangalam B, Lin P, Tam Y, Lin P, Weissman D, Kutzler M, Alameh MG, Haddad E. Research square. 2022 Nov 7 -
Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination.
Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, Wu Y, Behl S, Taylor JJ, Chakaraborty R, Johnson AJ, Shiavo DN, Utz JP, Reisenauer JS, Midthun DE, Mullon JJ, Edell ES, Alameh MG, Borish L, Teague WG, Kaplan MH, Weissman D, Kern R, Hu H, Vassallo R, Liu SL, Sun J. Science immunology. 2022 Oct 28;7(76):eadd4853 Epub 2022 Oct 21 -
Breadth of SARS-CoV-2 neutralization and protection induced by a nanoparticle vaccine.
Li D, Martinez DR, Schäfer A, Chen H, Barr M, Sutherland LL, Lee E, Parks R, Mielke D, Edwards W, Newman A, Bock KW, Minai M, Nagata BM, Gagne M, Douek DC, DeMarco CT, Denny TN, Oguin TH 3rd, Brown A, Rountree W, Wang Y, Mansouri K, Edwards RJ, Ferrari G, Sempowski GD, Eaton A, Tang J, Cain DW, Santra S, Pardi N, Weissman D, Tomai MA, Fox CB, Moore IN, Andersen H, Lewis MG, Golding H, Seder R, Khurana S, Baric RS, Montefiori DC, Saunders KO, Haynes BF. Nature communications. 2022 Oct 23;13(1):6309 -
mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model.
Sittplangkoon C, Alameh MG, Weissman D, Lin PJC, Tam YK, Prompetchara E, Palaga T. Frontiers in immunology. 2022;13:983000 Epub 2022 Oct 14 -
Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models.
Hajnik RL, Plante JA, Liang Y, Alameh MG, Tang J, Bonam SR, Zhong C, Adam A, Scharton D, Rafael GH, Liu Y, Hazell NC, Sun J, Soong L, Shi PY, Wang T, Walker DH, Sun J, Weissman D, Weaver SC, Plante KS, Hu H. Science translational medicine. 2022 Sep 14;14(662):eabq1945 -
Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells.
Patel SK, Billingsley MM, Frazee C, Han X, Swingle KL, Qin J, Alameh MG, Wang K, Weissman D, Mitchell MJ. Journal of controlled release : official journal of the Controlled Release Society. 2022 Jul;347:521-532 Epub 2022 May 23 -
Rational Design of Bisphosphonate Lipid-like Materials for mRNA Delivery to the Bone Microenvironment.
Xue L, Gong N, Shepherd SJ, Xiong X, Liao X, Han X, Zhao G, Song C, Huang X, Zhang H, Padilla MS, Qin J, Shi Y, Alameh MG, Pochan DJ, Wang K, Long F, Weissman D, Mitchell MJ. Journal of the American Chemical Society. 2022 Jun 8;144(22):9926-9937 Epub 2022 May 26 -
Rational design of anti-inflammatory lipid nanoparticles for mRNA delivery.
Zhang H, Han X, Alameh MG, Shepherd SJ, Padilla MS, Xue L, Butowska K, Weissman D, Mitchell MJ. Journal of biomedical materials research. Part A. 2022 May;110(5):1101-1108 Epub 2022 Jan 25 -
Development of mRNA manufacturing for vaccines and therapeutics: mRNA platform requirements and development of a scalable production process to support early phase clinical trials.
Whitley J, Zwolinski C, Denis C, Maughan M, Hayles L, Clarke D, Snare M, Liao H, Chiou S, Marmura T, Zoeller H, Hudson B, Peart J, Johnson M, Karlsson A, Wang Y, Nagle C, Harris C, Tonkin D, Fraser S, Capiz L, Zeno CL, Meli Y, Martik D, Ozaki DA, Caparoni A, Dickens JE, Weissman D, Saunders KO, Haynes BF, Sempowski GD, Denny TN, Johnson MR. Translational research : the journal of laboratory and clinical medicine. 2022 Apr;242:38-55 Epub 2021 Dec 4 -
Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE).
Parhiz H, Brenner JS, Patel PN, Papp TE, Shahnawaz H, Li Q, Shi R, Zamora ME, Yadegari A, Marcos-Contreras OA, Natesan A, Pardi N, Shuvaev VV, Kiseleva R, Myerson JW, Uhler T, Riley RS, Han X, Mitchell MJ, Lam K, Heyes J, Weissman D, Muzykantov VR. Journal of controlled release : official journal of the Controlled Release Society. 2022 Apr;344:50-61 Epub 2021 Dec 23 -
The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity.
Zhang D, Atochina-Vasserman EN, Lu J, Maurya DS, Xiao Q, Liu M, Adamson J, Ona N, Reagan EK, Ni H, Weissman D, Percec V. Journal of the American Chemical Society. 2022 Mar 23;144(11):4746-4753 Epub 2022 Mar 9 -
mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice.
Mu Z, Wiehe K, Saunders KO, Henderson R, Cain DW, Parks R, Martik D, Mansouri K, Edwards RJ, Newman A, Lu X, Xia SM, Eaton A, Bonsignori M, Montefiori D, Han Q, Venkatayogi S, Evangelous T, Wang Y, Rountree W, Korber B, Wagh K, Tam Y, Barbosa C, Alam SM, Williams WB, Tian M, Alt FW, Pardi N, Weissman D, Haynes BF. Cell reports. 2022 Mar 15;38(11):110514 -
Antibodies to Crucial Epitopes on HSV-2 Glycoprotein D as a Guide to Dosing an mRNA Genital Herpes Vaccine.
Hook LM, Awasthi S, Cairns TM, Alameh MG, Fowler BT, Egan KP, Sung MMH, Weissman D, Cohen GH, Friedman HM. Viruses. 2022 Mar 5;14(3) -
Breadth of SARS-CoV-2 Neutralization and Protection Induced by a Nanoparticle Vaccine.
Li D, Martinez DR, Schäfer A, Chen H, Barr M, Sutherland LL, Lee E, Parks R, Mielke D, Edwards W, Newman A, Bock KW, Minai M, Nagata BM, Gagne M, Douek DC, DeMarco CT, Denny TN, Oguin TH 3rd, Brown A, Rountree W, Wang Y, Mansouri K, Edwards RJ, Ferrari G, Sempowski GD, Eaton A, Tang J, Cain DW, Santra S, Pardi N, Weissman D, Tomai MA, Fox CB, Moore IN, Andersen H, Lewis MG, Golding H, Seder R, Khurana S, Baric RS, Montefiori DC, Saunders KO, Haynes BF. bioRxiv : the preprint server for biology. 2022 Feb 14 -
Nucleoside-Modified mRNA Vaccines Protect IFNAR(-/-) Mice against Crimean-Congo Hemorrhagic Fever Virus Infection.
Appelberg S, John L, Pardi N, Végvári Á, Bereczky S, Ahlén G, Monteil V, Abdurahman S, Mikaeloff F, Beattie M, Tam Y, Sällberg M, Neogi U, Weissman D, Mirazimi A. Journal of virology. 2022 Feb 9;96(3):e0156821 Epub 2021 Nov 24 -
CAR T cells produced in vivo to treat cardiac injury.
Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, Kimura T, Soliman OY, Papp TE, Tam YK, Mui BL, Albelda SM, Puré E, June CH, Aghajanian H, Weissman D, Parhiz H, Epstein JA. Science (New York, N.Y.). 2022 Jan 7;375(6576):91-96 Epub 2022 Jan 6 -
Messenger RNA-Based Vaccines Against Infectious Diseases.
Alameh MG, Weissman D, Pardi N. Current topics in microbiology and immunology. 2022;440:111-145 -
Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery.
Swingle KL, Billingsley MM, Bose SK, White B, Palanki R, Dave A, Patel SK, Gong N, Hamilton AG, Alameh MG, Weissman D, Peranteau WH, Mitchell MJ. Journal of controlled release : official journal of the Controlled Release Society. 2022 Jan;341:616-633 Epub 2021 Nov 3 -
Lipid nanoparticle chemistry determines how nucleoside base modifications alter mRNA delivery.
Melamed JR, Hajj KA, Chaudhary N, Strelkova D, Arral ML, Pardi N, Alameh MG, Miller JB, Farbiak L, Siegwart DJ, Weissman D, Whitehead KA. Journal of controlled release : official journal of the Controlled Release Society. 2022 Jan;341:206-214 Epub 2021 Nov 18 -
Is France Once Again Looking for a Scapegoat?
Lederman MM, Flier JS, Hale P, Haase AT, Powderly W, Reiss P, Silvestri G, Sekaly RP, Paiardini M, Weissman D, Kuritzkes DR, Calabrese LH, Agre P, Reyes-Teran G, Landay AL, Lewin S, Richman DD, Volberding P, Hunt PW, Schechter M. Pathogens & immunity. 2021;6(2):149-152 Epub 2021 Dec 29 -
Tick immunity using mRNA, DNA and protein-based Salp14 delivery strategies.
Matias J, Kurokawa C, Sajid A, Narasimhan S, Arora G, Diktas H, Lynn GE, DePonte K, Pardi N, Valenzuela JG, Weissman D, Fikrig E. Vaccine. 2021 Dec 20;39(52):7661-7668 Epub 2021 Nov 30 -
Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses.
Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, Gaudette BT, Soliman OY, Pine M, Hicks P, Manzoni TB, Knox JJ, Johnson JL, Laczkó D, Muramatsu H, Davis B, Meng W, Rosenfeld AM, Strohmeier S, Lin PJC, Mui BL, Tam YK, Karikó K, Jacquet A, Krammer F, Bates P, Cancro MP, Weissman D, Luning Prak ET, Allman D, Locci M, Pardi N. Immunity. 2021 Dec 14;54(12):2877-2892.e7 Epub 2021 Nov 4 -
An ionizable lipid toolbox for RNA delivery.
Han X, Zhang H, Butowska K, Swingle KL, Alameh MG, Weissman D, Mitchell MJ. Nature communications. 2021 Dec 13;12(1):7233 -
Trivalent nucleoside-modified mRNA vaccine yields durable memory B cell protection against genital herpes in preclinical models.
Awasthi S, Knox JJ, Desmond A, Alameh MG, Gaudette BT, Lubinski JM, Naughton A, Hook LM, Egan KP, Tam YK, Pardi N, Allman D, Luning Prak ET, Cancro MP, Weissman D, Cohen GH, Friedman HM. The Journal of clinical investigation. 2021 Dec 1;131(23) -
mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent.
Sajid A, Matias J, Arora G, Kurokawa C, DePonte K, Tang X, Lynn G, Wu MJ, Pal U, Strank NO, Pardi N, Narasimhan S, Weissman D, Fikrig E. Science translational medicine. 2021 Nov 17;13(620):eabj9827 -
Author Correction: Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates.
Saunders KO, Pardi N, Parks R, Santra S, Mu Z, Sutherland L, Scearce R, Barr M, Eaton A, Hernandez G, Goodman D, Hogan MJ, Tombacz I, Gordon DN, Rountree RW, Wang Y, Lewis MG, Pierson TC, Barbosa C, Tam Y, Matyas GR, Rao M, Beck Z, Shen X, Ferrari G, Tomaras GD, Montefiori DC, Weissman D, Haynes BF. NPJ vaccines. 2021 Nov 6;6(1):136 -
Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs.
Tombácz I, Laczkó D, Shahnawaz H, Muramatsu H, Natesan A, Yadegari A, Papp TE, Alameh MG, Shuvaev V, Mui BL, Tam YK, Muzykantov V, Pardi N, Weissman D, Parhiz H. Molecular therapy : the journal of the American Society of Gene Therapy. 2021 Nov 3;29(11):3293-3304 Epub 2021 Jun 4 -
Targeted Delivery of mRNA with One-Component Ionizable Amphiphilic Janus Dendrimers.
Zhang D, Atochina-Vasserman EN, Maurya DS, Liu M, Xiao Q, Lu J, Lauri G, Ona N, Reagan EK, Ni H, Weissman D, Percec V. Journal of the American Chemical Society. 2021 Nov 3;143(43):17975-17982 Epub 2021 Oct 21 -
mRNA vaccines for infectious diseases: principles, delivery and clinical translation.
Chaudhary N, Weissman D, Whitehead KA. Nature reviews. Drug discovery. 2021 Nov;20(11):817-838 Epub 2021 Aug 25 -
Author Correction: mRNA vaccines for infectious diseases: principles, delivery and clinical translation.
Chaudhary N, Weissman D, Whitehead KA. Nature reviews. Drug discovery. 2021 Nov;20(11):880 -
Transient yet Robust Expression of Proteins in the Mouse Liver via Intravenous Injection of Lipid Nanoparticle-encapsulated Nucleoside-modified mRNA.
Everton E, Rizvi F, Smith AR, Beattie M, Tam Y, Pardi N, Weissman D, Gouon-Evans V. Bio-protocol. 2021 Oct 5;11(19):e4184 -
Lipid-nanoparticle-encapsulated mRNA vaccines induce protective memory CD8 T cells against a lethal viral infection.
Knudson CJ, Alves-Peixoto P, Muramatsu H, Stotesbury C, Tang L, Lin PJC, Tam YK, Weissman D, Pardi N, Sigal LJ. Molecular therapy : the journal of the American Society of Gene Therapy. 2021 Sep 1;29(9):2769-2781 Epub 2021 May 14 -
Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice.
Martinez DR, Schäfer A, Leist SR, De la Cruz G, West A, Atochina-Vasserman EN, Lindesmith LC, Pardi N, Parks R, Barr M, Li D, Yount B, Saunders KO, Weissman D, Haynes BF, Montgomery SA, Baric RS. Science (New York, N.Y.). 2021 Aug 27;373(6558):991-998 Epub 2021 Jun 22 -
One-Component Multifunctional Sequence-Defined Ionizable Amphiphilic Janus Dendrimer Delivery Systems for mRNA.
Zhang D, Atochina-Vasserman EN, Maurya DS, Huang N, Xiao Q, Ona N, Liu M, Shahnawaz H, Ni H, Kim K, Billingsley MM, Pochan DJ, Mitchell MJ, Weissman D, Percec V. Journal of the American Chemical Society. 2021 Aug 11;143(31):12315-12327 Epub 2021 Jul 29 -
Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration.
Carrasco MJ, Alishetty S, Alameh MG, Said H, Wright L, Paige M, Soliman O, Weissman D, Cleveland TE 4th, Grishaev A, Buschmann MD. Communications biology. 2021 Aug 11;4(1):956 -
Ability of nucleoside-modified mRNA to encode HIV-1 envelope trimer nanoparticles.
Mu Z, Wiehe K, Saunders KO, Henderson R, Cain DW, Parks R, Martik D, Mansouri K, Edwards RJ, Newman A, Lu X, Xia SM, Bonsignori M, Montefiori D, Han Q, Venkatayogi S, Evangelous T, Wang Y, Rountree W, Tam Y, Barbosa C, Alam SM, Williams WB, Pardi N, Weissman D, Haynes BF. bioRxiv : the preprint server for biology. 2021 Aug 9 -
In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels.
Rothgangl T, Dennis MK, Lin PJC, Oka R, Witzigmann D, Villiger L, Qi W, Hruzova M, Kissling L, Lenggenhager D, Borrelli C, Egli S, Frey N, Bakker N, Walker JA 2nd, Kadina AP, Victorov DV, Pacesa M, Kreutzer S, Kontarakis Z, Moor A, Jinek M, Weissman D, Stoffel M, van Boxtel R, Holden K, Pardi N, Thöny B, Häberle J, Tam YK, Semple SC, Schwank G. Nature biotechnology. 2021 Aug;39(8):949-957 Epub 2021 May 19 -
Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device.
Shepherd SJ, Warzecha CC, Yadavali S, El-Mayta R, Alameh MG, Wang L, Weissman D, Wilson JM, Issadore D, Mitchell MJ. Nano letters. 2021 Jul 14;21(13):5671-5680 Epub 2021 Jun 30 -
Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice.
Mallory KL, Taylor JA, Zou X, Waghela IN, Schneider CG, Sibilo MQ, Punde NM, Perazzo LC, Savransky T, Sedegah M, Dutta S, Janse CJ, Pardi N, Lin PJC, Tam YK, Weissman D, Angov E. NPJ vaccines. 2021 Jun 18;6(1):84 -
Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema.
Szőke D, Kovács G, Kemecsei É, Bálint L, Szoták-Ajtay K, Aradi P, Styevkóné Dinnyés A, Mui BL, Tam YK, Madden TD, Karikó K, Kataru RP, Hope MJ, Weissman D, Mehrara BJ, Pardi N, Jakus Z. Nature communications. 2021 Jun 8;12(1):3460 -
Debunking mRNA Vaccine Misconceptions-An Overview for Medical Professionals.
Hitti FL, Weissman D. The American journal of medicine. 2021 Jun;134(6):703-704 Epub 2021 Mar 15 -
Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses.
Saunders KO, Lee E, Parks R, Martinez DR, Li D, Chen H, Edwards RJ, Gobeil S, Barr M, Mansouri K, Alam SM, Sutherland LL, Cai F, Sanzone AM, Berry M, Manne K, Bock KW, Minai M, Nagata BM, Kapingidza AB, Azoitei M, Tse LV, Scobey TD, Spreng RL, Rountree RW, DeMarco CT, Denny TN, Woods CW, Petzold EW, Tang J, Oguin TH 3rd, Sempowski GD, Gagne M, Douek DC, Tomai MA, Fox CB, Seder R, Wiehe K, Weissman D, Pardi N, Golding H, Khurana S, Acharya P, Andersen H, Lewis MG, Moore IN, Montefiori DC, Baric RS, Haynes BF. Nature. 2021 Jun;594(7864):553-559 Epub 2021 May 10 -
Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice.
Martinez DR, Schäfer A, Leist SR, De la Cruz G, West A, Atochina-Vasserman EN, Lindesmith LC, Pardi N, Parks R, Barr M, Li D, Yount B, Saunders KO, Weissman D, Haynes BF, Montgomery SA, Baric RS. bioRxiv : the preprint server for biology. 2021 May 11 -
Author Correction: Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA.
Rizvi F, Everton E, Smith AR, Liu H, Osota E, Beattie M, Tam Y, Pardi N, Weissman D, Gouon-Evans V. Nature communications. 2021 May 10;12(1):2825 -
Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates.
Saunders KO, Pardi N, Parks R, Santra S, Mu Z, Sutherland L, Scearce R, Barr M, Eaton A, Hernandez G, Goodman D, Hogan MJ, Tombacz I, Gordon DN, Rountree RW, Wang Y, Lewis MG, Pierson TC, Barbosa C, Tam Y, Matyas GR, Rao M, Beck Z, Shen X, Ferrari G, Tomaras GD, Montefiori DC, Weissman D, Haynes BF. NPJ vaccines. 2021 Apr 9;6(1):50 -
Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA.
Rizvi F, Everton E, Smith AR, Liu H, Osota E, Beattie M, Tam Y, Pardi N, Weissman D, Gouon-Evans V. Nature communications. 2021 Jan 27;12(1):613 -
Nanomaterial Delivery Systems for mRNA Vaccines.
Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D. Vaccines. 2021 Jan 19;9(1) -
D614G Spike Mutation Increases SARS CoV-2 Susceptibility to Neutralization.
Weissman D, Alameh MG, de Silva T, Collini P, Hornsby H, Brown R, LaBranche CC, Edwards RJ, Sutherland L, Santra S, Mansouri K, Gobeil S, McDanal C, Pardi N, Hengartner N, Lin PJC, Tam Y, Shaw PA, Lewis MG, Boesler C, Şahin U, Acharya P, Haynes BF, Korber B, Montefiori DC. Cell host & microbe. 2021 Jan 13;29(1):23-31.e4 Epub 2020 Dec 1 -
Ionizable lipid nanoparticles for in utero mRNA delivery.
Riley RS, Kashyap MV, Billingsley MM, White B, Alameh MG, Bose SK, Zoltick PW, Li H, Zhang R, Cheng AY, Weissman D, Peranteau WH, Mitchell MJ. Science advances. 2021 Jan;7(3) Epub 2021 Jan 13 -
Vaccination with Messenger RNA: A Promising Alternative to DNA Vaccination.
Tombácz I, Weissman D, Pardi N. Methods in molecular biology (Clifton, N.J.). 2021;2197:13-31 -
Safe and Effective In Vivo Targeting and Gene Editing in Hematopoietic Stem Cells: Strategies for Accelerating Development.
Cannon P, Asokan A, Czechowicz A, Hammond P, Kohn DB, Lieber A, Malik P, Marks P, Porteus M, Verhoeyen E, Weissman D, Weissman I, Kiem HP. Human gene therapy. 2021 Jan;32(1-2):31-42 -
SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation.
Lederer K, Castaño D, Gómez Atria D, Oguin TH 3rd, Wang S, Manzoni TB, Muramatsu H, Hogan MJ, Amanat F, Cherubin P, Lundgreen KA, Tam YK, Fan SHY, Eisenlohr LC, Maillard I, Weissman D, Bates P, Krammer F, Sempowski GD, Pardi N, Locci M. Immunity. 2020 Dec 15;53(6):1281-1295.e5 Epub 2020 Nov 21 -
Development of vaccines and antivirals for combating viral pandemics.
Pardi N, Weissman D. Nature biomedical engineering. 2020 Dec;4(12):1128-1133 -
Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mRNA in lipid nanoparticle vaccine.
LaTourette PC 2nd, Awasthi S, Desmond A, Pardi N, Cohen GH, Weissman D, Friedman HM. Vaccine. 2020 Nov 3;38(47):7409-7413 Epub 2020 Oct 9 -
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice.
Laczkó D, Hogan MJ, Toulmin SA, Hicks P, Lederer K, Gaudette BT, Castaño D, Amanat F, Muramatsu H, Oguin TH 3rd, Ojha A, Zhang L, Mu Z, Parks R, Manzoni TB, Roper B, Strohmeier S, Tombácz I, Arwood L, Nachbagauer R, Karikó K, Greenhouse J, Pessaint L, Porto M, Putman-Taylor T, Strasbaugh A, Campbell TA, Lin PJC, Tam YK, Sempowski GD, Farzan M, Choe H, Saunders KO, Haynes BF, Andersen H, Eisenlohr LC, Weissman D, Krammer F, Bates P, Allman D, Locci M, Pardi N. Immunity. 2020 Oct 13;53(4):724-732.e7 Epub 2020 Jul 30 -
A Common NLRC4 Gene Variant Associates With Inflammation and Pulmonary Function in Human Immunodeficiency Virus and Tuberculosis.
Ravimohan S, Maenetje P, Auld SC, Ncube I, Mlotshwa M, Chase W, Tiemessen CT, Vangu MD, Wallis RS, Churchyard G, Weissman D, Kornfeld H, Bisson GP. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Aug 14;71(4):924-932 -
Recent advances in mRNA vaccine technology.
Pardi N, Hogan MJ, Weissman D. Current opinion in immunology. 2020 Aug;65:14-20 Epub 2020 Mar 31 -
An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection.
Egan KP, Hook LM, Naughton A, Pardi N, Awasthi S, Cohen GH, Weissman D, Friedman HM. PLoS pathogens. 2020 Jul;16(7):e1008795 Epub 2020 Jul 27 -
A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice.
Freyn AW, Ramos da Silva J, Rosado VC, Bliss CM, Pine M, Mui BL, Tam YK, Madden TD, de Souza Ferreira LC, Weissman D, Krammer F, Coughlan L, Palese P, Pardi N, Nachbagauer R. Molecular therapy : the journal of the American Society of Gene Therapy. 2020 Jul 8;28(7):1569-1584 Epub 2020 Apr 19 -
Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria.
Raj DK, Das Mohapatra A, Jnawali A, Zuromski J, Jha A, Cham-Kpu G, Sherman B, Rudlaff RM, Nixon CE, Hilton N, Oleinikov AV, Chesnokov O, Merritt J, Pond-Tor S, Burns L, Jolly G, Ben Mamoun C, Kabyemela E, Muehlenbachs A, Lambert L, Orr-Gonzalez S, Gnädig NF, Fidock DA, Park S, Dvorin JD, Pardi N, Weissman D, Mui BL, Tam YK, Friedman JF, Fried M, Duffy PE, Kurtis JD. Nature. 2020 Jun;582(7810):104-108 Epub 2020 Apr 22 -
The Transcription Factor T-bet Resolves Memory B Cell Subsets with Distinct Tissue Distributions and Antibody Specificities in Mice and Humans.
Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, Kostiv M, Rosenfeld AM, Meng W, Christensen SR, Hensley SE, Yewdell J, Canaday DH, Zhu J, McDermott AB, Dori Y, Itkin M, Wherry EJ, Pardi N, Weissman D, Naji A, Prak ETL, Betts MR, Cancro MP. Immunity. 2020 May 19;52(5):842-855.e6 Epub 2020 Apr 29 -
Evaluation of a Single-Dose Nucleoside-Modified Messenger RNA Vaccine Encoding Hendra Virus-Soluble Glycoprotein Against Lethal Nipah virus Challenge in Syrian Hamsters.
Lo MK, Spengler JR, Welch SR, Harmon JR, Coleman-McCray JD, Scholte FEM, Shrivastava-Ranjan P, Montgomery JM, Nichol ST, Weissman D, Spiropoulou CF. The Journal of infectious diseases. 2020 May 11;221(Suppl 4):S493-S498 -
Human Cytomegalovirus Glycoprotein B Nucleoside-Modified mRNA Vaccine Elicits Antibody Responses with Greater Durability and Breadth than MF59-Adjuvanted gB Protein Immunization.
Nelson CS, Jenks JA, Pardi N, Goodwin M, Roark H, Edwards W, McLellan JS, Pollara J, Weissman D, Permar SR. Journal of virology. 2020 Apr 16;94(9) -
Lung Injury on Antiretroviral Therapy in Adults With Human Immunodeficiency Virus/Tuberculosis.
Ravimohan S, Auld SC, Maenetje P, Ratsela N, Mlotshwa M, Ncube I, Smith JP, Vangu MD, Sebe M, Kossenkov A, Weissman D, Wallis RS, Churchyard G, Kornfeld H, Bisson GP. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Apr 15;70(9):1845-1854 -
Declines in Lung Function After Antiretroviral Therapy Initiation in Adults With Human Immunodeficiency Virus and Tuberculosis: A Potential Manifestation of Respiratory Immune Reconstitution Inflammatory Syndrome.
Auld SC, Maenetje P, Ravimohan S, Weissman D, Ncube I, Mlotshwa M, Ratsela N, Chase W, Vangu MD, Wallis R, Churchyard G, Kornfeld H, Bisson GP. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Apr 10;70(8):1750-1753 -
Vaccine innovations for emerging infectious diseases-a symposium report.
Cable J, Srikantiah P, Crowe JE Jr, Pulendran B, Hill A, Ginsberg A, Koff W, Mathew A, Ng T, Jansen K, Glenn G, Permar S, Wilson I, Weiner DB, Weissman D, Rappuoli R. Annals of the New York Academy of Sciences. 2020 Feb;1462(1):14-26 Epub 2019 Oct 28 -
Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice.
Willis E, Pardi N, Parkhouse K, Mui BL, Tam YK, Weissman D, Hensley SE. Science translational medicine. 2020 Jan 8;12(525) -
Optimizing ethambutol dosing among HIV/tuberculosis co-infected patients: a population pharmacokinetic modelling and simulation study.
Mehta K, Ravimohan S, Pasipanodya JG, Srivastava S, Modongo C, Zetola NM, Weissman D, Ivaturi V, Gumbo T, Bisson GP, Vinnard C. The Journal of antimicrobial chemotherapy. 2019 Oct 1;74(10):2994-3002 -
Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes.
Awasthi S, Hook LM, Pardi N, Wang F, Myles A, Cancro MP, Cohen GH, Weissman D, Friedman HM. Science immunology. 2019 Sep 20;4(39) -
Characterization of HIV-1 Nucleoside-Modified mRNA Vaccines in Rabbits and Rhesus Macaques.
Pardi N, LaBranche CC, Ferrari G, Cain DW, Tombácz I, Parks RJ, Muramatsu H, Mui BL, Tam YK, Karikó K, Polacino P, Barbosa CJ, Madden TD, Hope MJ, Haynes BF, Montefiori DC, Hu SL, Weissman D. Molecular therapy. Nucleic acids. 2019 Apr 15;15:36-47 Epub 2019 Mar 21 -
Purification of mRNA Encoding Chimeric Antigen Receptor Is Critical for Generation of a Robust T-Cell Response.
Foster JB, Choudhari N, Perazzelli J, Storm J, Hofmann TJ, Jain P, Storm PB, Pardi N, Weissman D, Waanders AJ, Grupp SA, Karikó K, Resnick AC, Barrett DM. Human gene therapy. 2019 Feb;30(2):168-178 Epub 2018 Oct 2 -
PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake.
Parhiz H, Shuvaev VV, Pardi N, Khoshnejad M, Kiseleva RY, Brenner JS, Uhler T, Tuyishime S, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D, Muzykantov VR. Journal of controlled release : official journal of the Controlled Release Society. 2018 Dec 10;291:106-115 Epub 2018 Oct 15 -
Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies.
Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, Tam YK, Karikó K, Barbosa CJ, Madden TD, Hope MJ, Krammer F, Hensley SE, Weissman D. Nature communications. 2018 Aug 22;9(1):3361 -
Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses.
Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, Moody MA, Verkerke HP, Myles A, Willis E, LaBranche CC, Montefiori DC, Lobby JL, Saunders KO, Liao HX, Korber BT, Sutherland LL, Scearce RM, Hraber PT, Tombácz I, Muramatsu H, Ni H, Balikov DA, Li C, Mui BL, Tam YK, Krammer F, Karikó K, Polacino P, Eisenlohr LC, Madden TD, Hope MJ, Lewis MG, Lee KK, Hu SL, Hensley SE, Cancro MP, Haynes BF, Weissman D. The Journal of experimental medicine. 2018 Jun 4;215(6):1571-1588 Epub 2018 May 8 -
Common Variation in NLRP3 Is Associated With Early Death and Elevated Inflammasome Biomarkers Among Advanced HIV/TB Co-infected Patients in Botswana.
Ravimohan S, Nfanyana K, Tamuhla N, Tiemessen CT, Weissman D, Bisson GP. Open forum infectious diseases. 2018 May;5(5):ofy075 Epub 2018 Apr 11 -
mRNA vaccines - a new era in vaccinology.
Pardi N, Hogan MJ, Porter FW, Weissman D. Nature reviews. Drug discovery. 2018 Apr;17(4):261-279 Epub 2018 Jan 12 -
Tuberculosis and lung damage: from epidemiology to pathophysiology.
Ravimohan S, Kornfeld H, Weissman D, Bisson GP. European respiratory review : an official journal of the European Respiratory Society. 2018 Mar 31;27(147) Epub 2018 Feb 28 -
Increased surface expression of HIV-1 envelope is associated with improved antibody response in vaccinia prime/protein boost immunization.
Hogan MJ, Conde-Motter A, Jordan APO, Yang L, Cleveland B, Guo W, Romano J, Ni H, Pardi N, LaBranche CC, Montefiori DC, Hu SL, Hoxie JA, Weissman D. Virology. 2018 Jan 15;514:106-117 Epub 2017 Nov 22 -
Pyrazinamide clearance is impaired among HIV/tuberculosis patients with high levels of systemic immune activation.
Vinnard C, Ravimohan S, Tamuhla N, Pasipanodya J, Srivastava S, Modongo C, Zetola NM, Weissman D, Gumbo T, Bisson GP. PloS one. 2017;12(11):e0187624 Epub 2017 Nov 2 -
Markers of gut dysfunction do not explain low rifampicin bioavailability in HIV-associated TB.
Vinnard C, Ravimohan S, Tamuhla N, Pasipanodya J, Srivastava S, Modongo C, Zetola NM, Weissman D, Gumbo T, Bisson GP. The Journal of antimicrobial chemotherapy. 2017 Jul 1;72(7):2020-2027 -
Elevated Pre-Antiretroviral Therapy CD39+CD8+ T Cell Frequency Is Associated With Early Mortality in Advanced Human Immunodeficiency Virus/Tuberculosis Co-infection.
Ravimohan S, Tamuhla N, Nfanyana K, Ni H, Steenhoff AP, Gross R, Weissman D, Bisson GP. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 May 15;64(10):1453-1456 -
Isoniazid clearance is impaired among human immunodeficiency virus/tuberculosis patients with high levels of immune activation.
Vinnard C, Ravimohan S, Tamuhla N, Ivaturi V, Pasipanodya J, Srivastava S, Modongo C, Zetola NM, Weissman D, Gumbo T, Bisson GP. British journal of clinical pharmacology. 2017 Apr;83(4):801-811 Epub 2016 Dec 9 -
Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination.
Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu JS, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang YJ, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Karikó K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D. Nature. 2017 Mar 9;543(7644):248-251 Epub 2017 Feb 2 -
Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge.
Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, Yi Y, Muramatsu H, Ni H, Mui BL, Tam YK, Shaheen F, Collman RG, Karikó K, Danet-Desnoyers GA, Madden TD, Hope MJ, Weissman D. Nature communications. 2017 Mar 2;8:14630 -
Nucleoside Modified mRNA Vaccines for Infectious Diseases.
Pardi N, Weissman D. Methods in molecular biology (Clifton, N.J.). 2017;1499:109-121 -
Measuring the Adjuvant Activity of RNA Vaccines.
Pardi N, Weissman D. Methods in molecular biology (Clifton, N.J.). 2017;1499:143-153 -
Robust Reconstitution of Tuberculosis-Specific Polyfunctional CD4+ T-Cell Responses and Rising Systemic Interleukin 6 in Paradoxical Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome.
Ravimohan S, Tamuhla N, Nfanyana K, Steenhoff AP, Letlhogile R, Frank I, MacGregor RR, Gross R, Weissman D, Bisson GP. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Mar 15;62(6):795-803 Epub 2015 Nov 26 -
Matrix Metalloproteinases in Tuberculosis-Immune Reconstitution Inflammatory Syndrome and Impaired Lung Function Among Advanced HIV/TB Co-infected Patients Initiating Antiretroviral Therapy.
Ravimohan S, Tamuhla N, Kung SJ, Nfanyana K, Steenhoff AP, Gross R, Weissman D, Bisson GP. EBioMedicine. 2016 Jan;3:100-107 Epub 2015 Nov 25 -
Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes.
Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D. Journal of controlled release : official journal of the Controlled Release Society. 2015 Nov 10;217:345-51 Epub 2015 Aug 8 -
mRNA: Fulfilling the Promise of Gene Therapy.
Weissman D, Karikó K. Molecular therapy : the journal of the American Society of Gene Therapy. 2015 Sep;23(9):1416-7 -
Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils.
Castellano LM, Hammond RM, Holmes VM, Weissman D, Shorter J. Biology open. 2015 Aug 28;4(9):1206-12 -
Repurposing Hsp104 to Antagonize Seminal Amyloid and Counter HIV Infection.
Castellano LM, Bart SM, Holmes VM, Weissman D, Shorter J. Chemistry & biology. 2015 Aug 20;22(8):1074-86 Epub 2015 Aug 6 -
A molecular tweezer antagonizes seminal amyloids and HIV infection.
Lump E, Castellano LM, Meier C, Seeliger J, Erwin N, Sperlich B, Stürzel CM, Usmani S, Hammond RM, von Einem J, Gerold G, Kreppel F, Bravo-Rodriguez K, Pietschmann T, Holmes VM, Palesch D, Zirafi O, Weissman D, Sowislok A, Wettig B, Heid C, Kirchhoff F, Weil T, Klärner FG, Schrader T, Bitan G, Sanchez-Garcia E, Winter R, Shorter J, Münch J. eLife. 2015 Aug 18;4 -
Periluminal Distribution of HIV-Binding Target Cells and Gp340 in the Oral, Cervical and Sigmoid/Rectal Mucosae: A Mapping Study.
Patyka M, Malamud D, Weissman D, Abrams WR, Kurago Z. PloS one. 2015;10(7):e0132942 Epub 2015 Jul 14 -
Identification of Cyclobutane Pyrimidine Dimer-Responsive Genes Using UVB-Irradiated Human Keratinocytes Transfected with In Vitro-Synthesized Photolyase mRNA.
Boros G, Miko E, Muramatsu H, Weissman D, Emri E, van der Horst GT, Szegedi A, Horkay I, Emri G, Karikó K, Remenyik É. PloS one. 2015;10(6):e0131141 Epub 2015 Jun 29 -
Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study.
Ravimohan S, Tamuhla N, Steenhoff AP, Letlhogile R, Nfanyana K, Bellamy SL, MacGregor RR, Gross R, Weissman D, Bisson GP. The Lancet. Infectious diseases. 2015 Apr;15(4):429-38 Epub 2015 Feb 9 -
Broad-spectrum antibiotic or G-CSF as potential countermeasures for impaired control of bacterial infection associated with an SPE exposure during spaceflight.
Li M, Holmes V, Ni H, Sanzari JK, Romero-Weaver AL, Lin L, Carabe-Fernandez A, Diffenderfer ES, Kennedy AR, Weissman D. PloS one. 2015;10(3):e0120126 Epub 2015 Mar 20 -
mRNA transcript therapy.
Weissman D. Expert review of vaccines. 2015 Feb;14(2):265-81 Epub 2014 Oct 31 -
Ionizing radiation selectively reduces skin regulatory T cells and alters immune function.
Zhou Y, Ni H, Balint K, Sanzari JK, Dentchev T, Diffenderfer ES, Wilson JM, Cengel KA, Weissman D. PloS one. 2014;9(6):e100800 Epub 2014 Jun 24 -
Hindlimb suspension and SPE-like radiation impairs clearance of bacterial infections.
Li M, Holmes V, Zhou Y, Ni H, Sanzari JK, Kennedy AR, Weissman D. PloS one. 2014;9(1):e85665 Epub 2014 Jan 15 -
Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis.
Bisson GP, Molefi M, Bellamy S, Thakur R, Steenhoff A, Tamuhla N, Rantleru T, Tsimako I, Gluckman S, Ravimohan S, Weissman D, Tebas P. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Apr;56(8):1165-73 Epub 2013 Jan 29 -
HPLC purification of in vitro transcribed long RNA.
Weissman D, Pardi N, Muramatsu H, Karikó K. Methods in molecular biology (Clifton, N.J.). 2013;969:43-54 -
In vitro transcription of long RNA containing modified nucleosides.
Pardi N, Muramatsu H, Weissman D, Karikó K. Methods in molecular biology (Clifton, N.J.). 2013;969:29-42 -
Upregulation of the phthiocerol dimycocerosate biosynthetic pathway by rifampin-resistant, rpoB mutant Mycobacterium tuberculosis.
Bisson GP, Mehaffy C, Broeckling C, Prenni J, Rifat D, Lun DS, Burgos M, Weissman D, Karakousis PC, Dobos K. Journal of bacteriology. 2012 Dec;194(23):6441-52 Epub 2012 Sep 21 -
Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin.
Karikó K, Muramatsu H, Keller JM, Weissman D. Molecular therapy : the journal of the American Society of Gene Therapy. 2012 May;20(5):948-53 Epub 2012 Feb 14 -
Effect of solar particle event radiation and hindlimb suspension on gastrointestinal tract bacterial translocation and immune activation.
Zhou Y, Ni H, Li M, Sanzari JK, Diffenderfer ES, Lin L, Kennedy AR, Weissman D. PloS one. 2012;7(9):e44329 Epub 2012 Sep 19 -
Nucleoside modifications in RNA limit activation of 2'-5'-oligoadenylate synthetase and increase resistance to cleavage by RNase L.
Anderson BR, Muramatsu H, Jha BK, Silverman RH, Weissman D, Karikó K. Nucleic acids research. 2011 Nov;39(21):9329-38 Epub 2011 Aug 3 -
Acute biological effects of simulating the whole-body radiation dose distribution from a solar particle event using a porcine model.
Wilson JM, Sanzari JK, Diffenderfer ES, Yee SS, Seykora JT, Maks C, Ware JH, Litt HI, Reetz JA, McDonough J, Weissman D, Kennedy AR, Cengel KA. Radiation research. 2011 Nov;176(5):649-59 Epub 2011 Aug 22 -
Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA.
Karikó K, Muramatsu H, Ludwig J, Weissman D. Nucleic acids research. 2011 Nov;39(21):e142 Epub 2011 Sep 2 -
SIV antigen immunization induces transient antigen-specific T cell responses and selectively activates viral replication in draining lymph nodes in retroviral suppressed rhesus macaques.
Hu H, Gama L, Aye PP, Clements JE, Barry PA, Lackner AA, Weissman D. Retrovirology. 2011 Jul 13;8:57 -
Effect of solar particle event radiation on gastrointestinal tract bacterial translocation and immune activation.
Ni H, Balint K, Zhou Y, Gridley DS, Maks C, Kennedy AR, Weissman D. Radiation research. 2011 Apr;175(4):485-92 Epub 2011 Feb 4 -
Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation.
Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D, Karikó K. Nucleic acids research. 2010 Sep;38(17):5884-92 Epub 2010 May 10 -
gp340 promotes transcytosis of human immunodeficiency virus type 1 in genital tract-derived cell lines and primary endocervical tissue.
Stoddard E, Ni H, Cannon G, Zhou C, Kallenbach N, Malamud D, Weissman D. Journal of virology. 2009 Sep;83(17):8596-603 Epub 2009 Jun 24 -
Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability.
Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Molecular therapy : the journal of the American Society of Gene Therapy. 2008 Nov;16(11):1833-40 Epub 2008 Sep 16 -
HIV envelope binding by macrophage-expressed gp340 promotes HIV-1 infection.
Cannon G, Yi Y, Ni H, Stoddard E, Scales DA, Van Ryk DI, Chaiken I, Malamud D, Weissman D. Journal of immunology (Baltimore, Md. : 1950). 2008 Aug 1;181(3):2065-70 -
HIV envelope suppresses CD4+ T cell activation independent of T regulatory cells.
Hu H, Fernando K, Ni H, Weissman D. Journal of immunology (Baltimore, Md. : 1950). 2008 Apr 15;180(8):5593-600 -
gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission.
Stoddard E, Cannon G, Ni H, Karikó K, Capodici J, Malamud D, Weissman D. Journal of immunology (Baltimore, Md. : 1950). 2007 Sep 1;179(5):3126-32 -
Naturally occurring nucleoside modifications suppress the immunostimulatory activity of RNA: implication for therapeutic RNA development.
Karikó K, Weissman D. Current opinion in drug discovery & development. 2007 Sep;10(5):523-32 -
Vaccine-delivered HIV envelope inhibits CD4(+) T-cell activation, a mechanism for poor HIV vaccine responses.
Fernando K, Hu H, Ni H, Hoxie JA, Weissman D. Blood. 2007 Mar 15;109(6):2538-44 Epub 2006 Dec 7 -
The N-terminal SRCR-SID domain of gp-340 interacts with HIV type 1 gp120 sequences and inhibits viral infection.
Wu Z, Lee S, Abrams W, Weissman D, Malamud D. AIDS research and human retroviruses. 2006 Jun;22(6):508-15 -
Effect of GB virus C viremia on HIV acquisition and HIV set-point.
Bisson GP, Strom BL, Gross R, Weissman D, Klinzman D, Hwang WT, Kostman JR, Metzger D, Stapleton JT, Frank I. AIDS (London, England). 2005 Nov 4;19(16):1910-2 -
Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA.
Karikó K, Buckstein M, Ni H, Weissman D. Immunity. 2005 Aug;23(2):165-75 -
Inhibition of toll-like receptor and cytokine signaling--a unifying theme in ischemic tolerance.
Karikó K, Weissman D, Welsh FA. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004 Nov;24(11):1288-304 -
Short interfering RNA-mediated inhibition of herpes simplex virus type 1 gene expression and function during infection of human keratinocytes.
Bhuyan PK, Karikò K, Capodici J, Lubinski J, Hook LM, Friedman HM, Weissman D. Journal of virology. 2004 Oct;78(19):10276-81 -
Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3.
Karikó K, Bhuyan P, Capodici J, Weissman D. Journal of immunology (Baltimore, Md. : 1950). 2004 Jun 1;172(11):6545-9 -
Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells.
Koski GK, Karikó K, Xu S, Weissman D, Cohen PA, Czerniecki BJ. Journal of immunology (Baltimore, Md. : 1950). 2004 Apr 1;172(7):3989-93 -
mRNA is an endogenous ligand for Toll-like receptor 3.
Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. The Journal of biological chemistry. 2004 Mar 26;279(13):12542-50 Epub 2004 Jan 16 -
Exogenous siRNA mediates sequence-independent gene suppression by signaling through toll-like receptor 3.
Karikó K, Bhuyan P, Capodici J, Ni H, Lubinski J, Friedman H, Weissman D. Cells, tissues, organs. 2004;177(3):132-8 -
Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR.
Lin G, Simmons G, Pöhlmann S, Baribaud F, Ni H, Leslie GJ, Haggarty BS, Bates P, Weissman D, Hoxie JA, Doms RW. Journal of virology. 2003 Jan;77(2):1337-46 -
RNA based vaccines.
Cannon G, Weissman D. DNA and cell biology. 2002 Dec;21(12):953-61 -
Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference.
Capodici J, Karikó K, Weissman D. Journal of immunology (Baltimore, Md. : 1950). 2002 Nov 1;169(9):5196-201 -
Immune reconstitution.
Weissman D, Montaner LJ. Clinics in laboratory medicine. 2002 Sep;22(3):719-40 -
Extracellular mRNA induces dendritic cell activation by stimulating tumor necrosis factor-alpha secretion and signaling through a nucleotide receptor.
Ni H, Capodici J, Cannon G, Communi D, Boeynaems JM, Karikó K, Weissman D. The Journal of biological chemistry. 2002 Apr 12;277(15):12689-96 Epub 2002 Jan 30



