Research
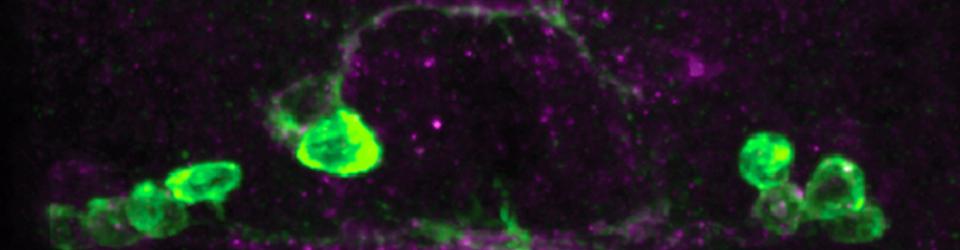
Our research is directed toward understanding fundamental mechanisms that control the development of the nervous system. We use the Drosophila embryonic CNS and the mouse spinal cord systems to study these mechanisms, with a particular emphasis on the control of axon guidance at the midline. Defects in the formation of midline circuitry can lead to profound deficits in motor control and cognitive abilities. For example, in the youtube video below, mutations in the DCC receptor can result in mirror movement disorders and intellectual disability.
Coordination of Commissural Axon Responses at the Midline
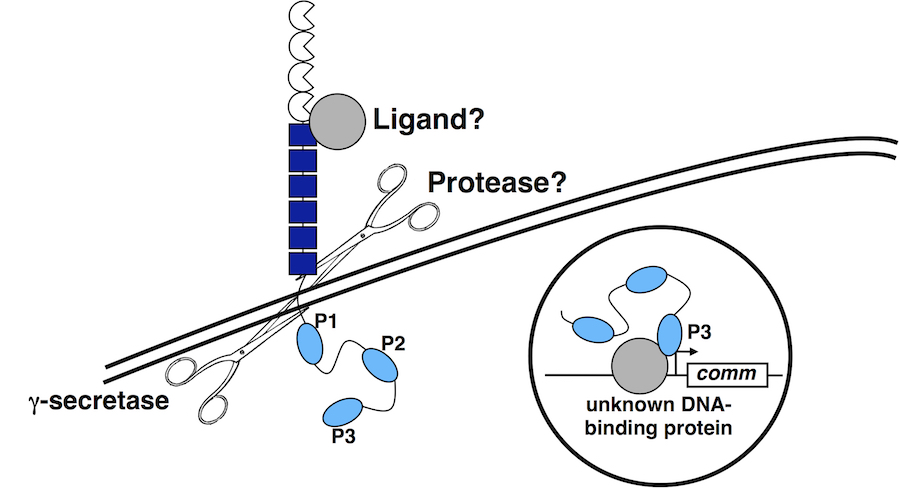
In order to cross the midline, commissural axons must down-regulate their responsiveness to the midline repellant Slit. In Drosophila, this is achieved by the Commissureless (Comm) protein, which prevents the Robo receptor from accumulating on the surface of pre-crossing commissural axons. comm mRNA expression is restricted to commissural neurons, where it is expressed in a pulse as axons are traversing the midline. We have discovered a role for Fra/DCC in the regulation of comm mRNA expression and have defined the mechanism through which Fra/DCC regulates transcription. Molecular, genetic, and biochemical evidence indicate that Fra is cleaved to release its intracellular domain (ICD), which shuttles between the cytoplasm and the nucleus, where it functions as a transcriptional activator. Rescue and gain-of-function experiments demonstrate that the Fra ICD is sufficient to regulate comm expression and that both g-secretase proteolysis of Fra and Fra’s function as a transcriptional activator are required for its ability to regulate comm in vivo. Our data uncover an unexpected role for the Fra ICD as a transcription factor whose activity regulates the responsiveness of commissural axons at the midline and raise the possibility that nuclear signaling may be a common output of axon guidance receptors.
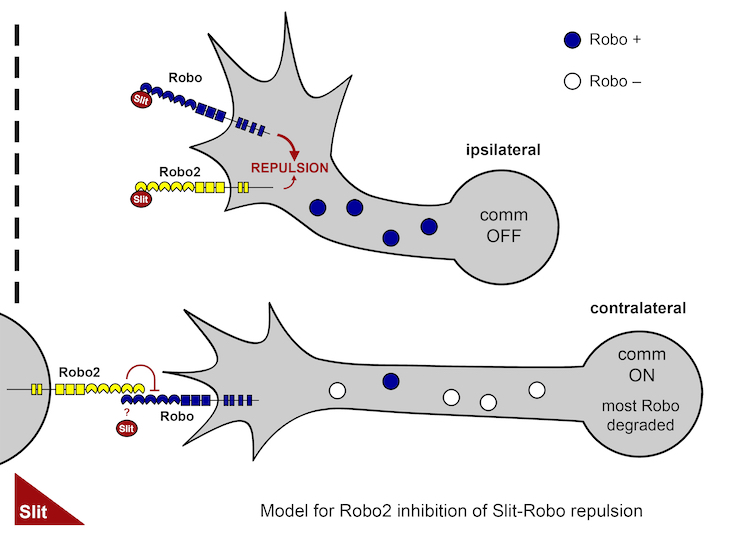
In a separate recent study, we have discovered an additional mechanism regulating commissural neuron responsiveness to midline Slit. Here we have found that the Robo2 receptor acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons. This study resolves the paradoxical ability of the Robo2 receptor to both promote and inhibit midline crossing of commissural axons.
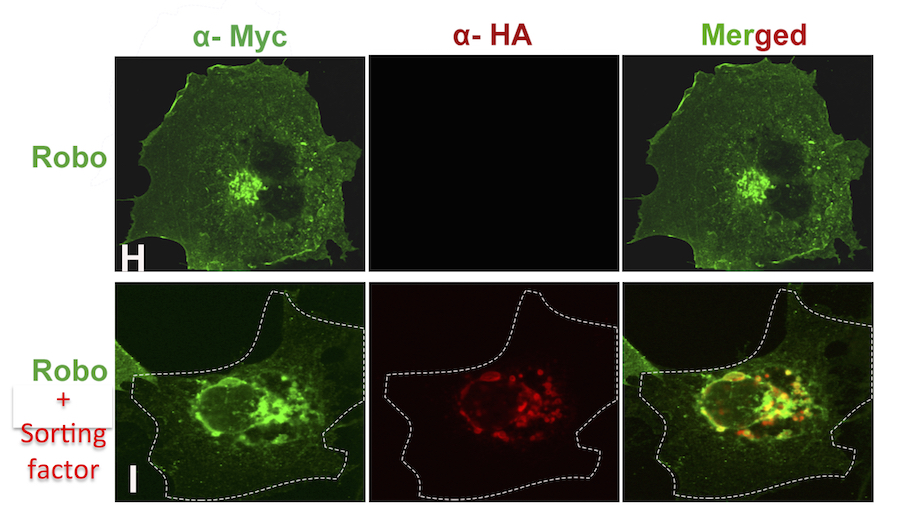
We are also investigating the extent to which these mechanisms are conserved in the mouse spinal cord. In ongoing work we have identified two mammalian proteins that function analogously to the fly Comm gene to direct the trafficking of mammalian Robo receptors. When co-expressed with Robo receptors in cultured mammalian cells, these sorting factors redirect Robo to the late endosome and trigger its degradation.
Close
Axon Guidance Receptor Signaling
Axon Guidance Receptor Signaling: Robo
Using genetic and molecular approaches, we are dissecting the signaling mechanisms that function downstream of Slit-Robo mediated axon repulsion. Our work on Robo receptor signaling has identified a number of conserved molecules that contribute to Robo repulsion. Genetic, biochemical and cell biological analyses support the existence of an evolutionary conserved signaling mechanism that links Robo receptors to the regulation of the Rho family small GTPases, and subsequent cytoskeletal reorganization. Specifically, activation of the Robo receptor by Slit leads to the rapid recruitment of the SH3-SH2 adaptor protein Dreadlocks, which in turn recruits the Ras/Rho Guanine Nucleotide exchange factor Son-of sevenless (Sos) to activate the Rac small GTPase during axon repulsion. We have also demonstrated that receptor proteolysis and endocytic trafficking are essential steps in Robo-mediated axon repulsion. A thorough understanding of how Robo receptors transmit extracellular signals to precicesly control axon repulsion will offer general insights into guidance receptors constrain growth both within and outside of the nervous system.
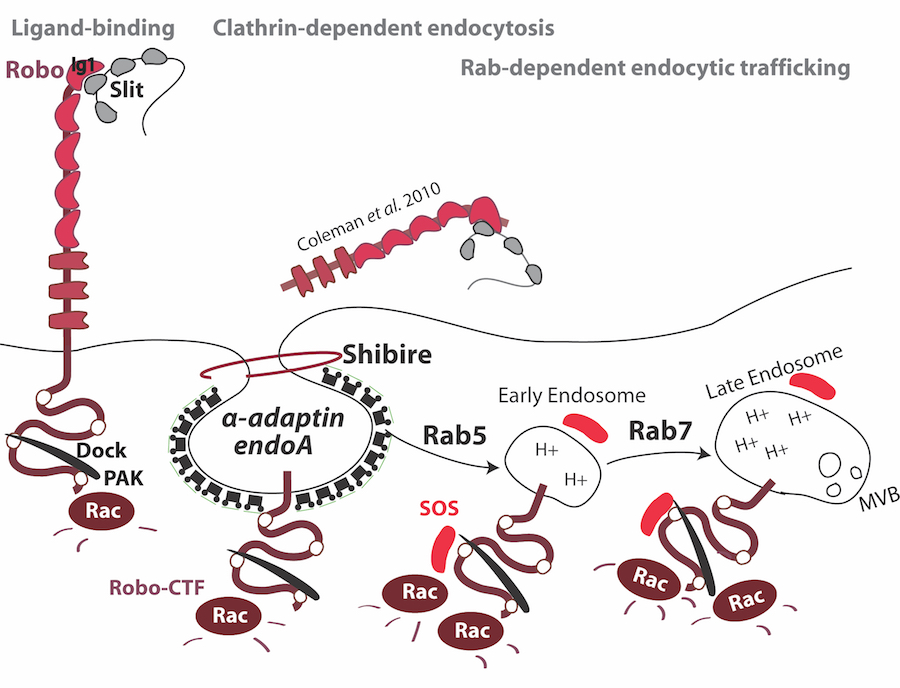
Axon Guidance Receptor Signaling: Frazzled/DCC
The DCC family of Netrin receptors regulates attractive axon guidance in C. elegans, Drosophila and mice. Netrin-dependent signaling functions are directed by the conserved cytoplasmic P1, P2 and P3 motifs, while other sequence elements recruit adapter and signaling molecules. We have dissected the in vivo requirement for these motifs in mediating attractive axon guidance. In addition, we have provided the first in vivo investigation of the role of Src-family kinases in Fra/DCC signaling and have identified additional players including the Abl tyrosine kinase in the Fra/DCC signaling pathway. Defining how DCC signals to promote neuronal growth may suggest strategies to promote repair and regeneration.
Axon Guidance Receptor Signaling: Transmembrane Semaphorins
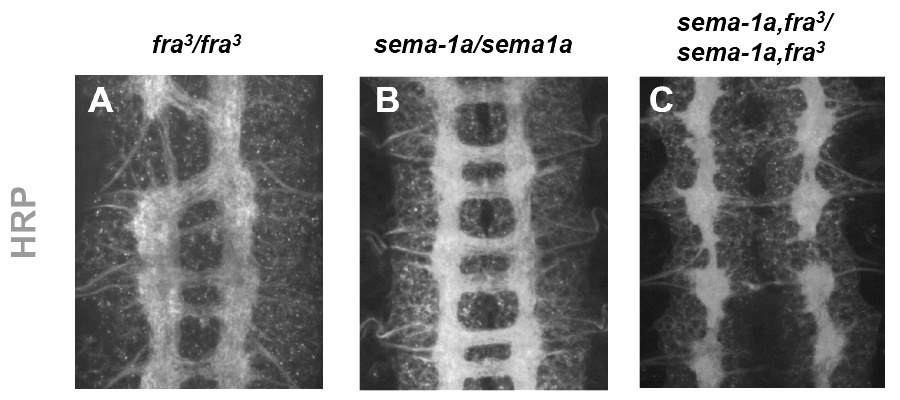
Although Netrin-Frazzled signaling plays an important role in guiding axons across the midline, many axons are still able to cross normally in the complete absence of Netrin and Frazzled, indicating that parallel pathways must exist. In a screen for genes that promote axon growth across the midline, we have identified a new role for the transmembrane Semaphorin, Sema1a, as an important signaling receptor for midline guidance. Our findings suggest, that in response to secreted Semaphorins produced at the midline, Sema1a acts in extending commissural axons to promote midline attraction by impinging on the activity of the Rho family small GTPase.
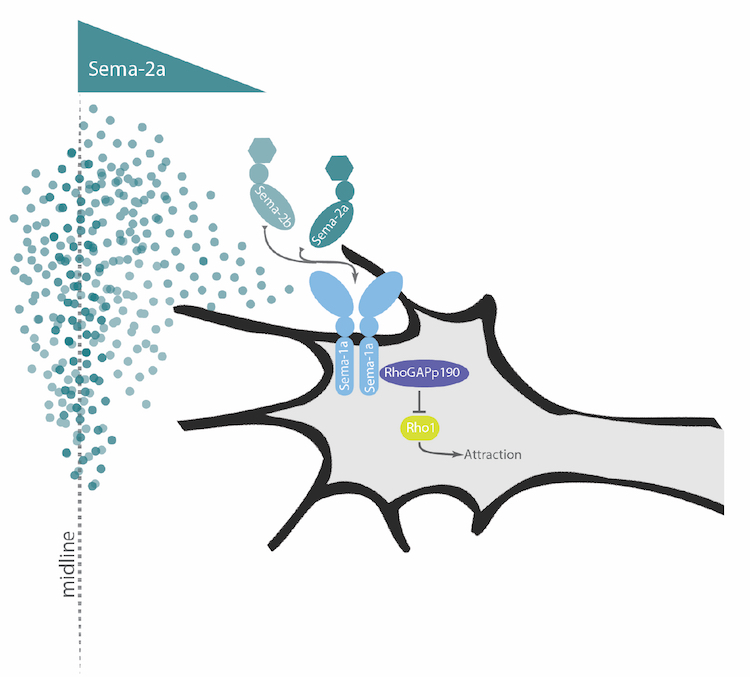
Intriguingly, in the absence of both Frazzled and Sema1a function, very few axons are able to cross the midline, a phenotype resembling loss of Commissureless gene function. These findings highlight the diversity of conserved molecules and mechanisms that contribute to the correct patterning of axonal connections at the midline.
Close
Diversification of Robo receptor function
Although axon guidance receptors- particularly in higher vertebrates- are often encoded by multiple closely related genes, whether and how these structurally related receptors differ in function is unclear. It is known that the three Drosophila Robo receptors contribute to at least three distinct guidance functions in the embryo: 1) Robo and Robo2 cooperate to mediate midline repulsion, 2) Robo2 and Robo3 regulate the medio-lateral position of longitudinal axons and 3) Robo2 can act to promote midline crossing in some contexts.
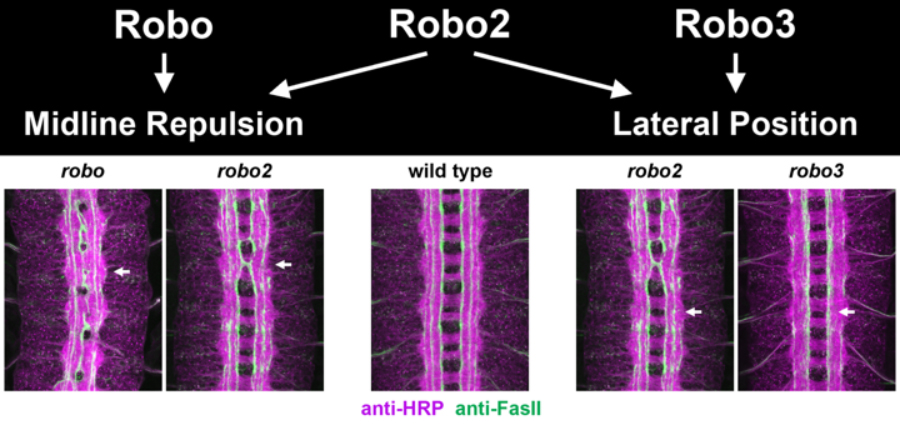
The extracellular domains of the three Robos share similar domain structures, including five immunoglobulin (Ig) domains and three fibronectin (FN) type III repeats. Robo intracellular domains are more divergent. Robo1 has four Conserved Cytoplasmic (CC) motifs- CC0, CC1, CC2 and CC3. Robo2 and Robo3 lack CC2 and CC3, the motifs most strongly implicated in repulsive signaling. These differences have led to the speculation that distinct Robo receptor functions would be directed by their divergent cytoplasmic domains. However, we have found that functional diversity of the Drosophila Robo receptors depends on an unexpected role for the extracellular Ig domains in conferring distinct biochemical properties to the Robos. Studies of the Drosophila Robos will continue to provide an important framework for understanding how guidance receptors diversify their activities to build complex circuits.
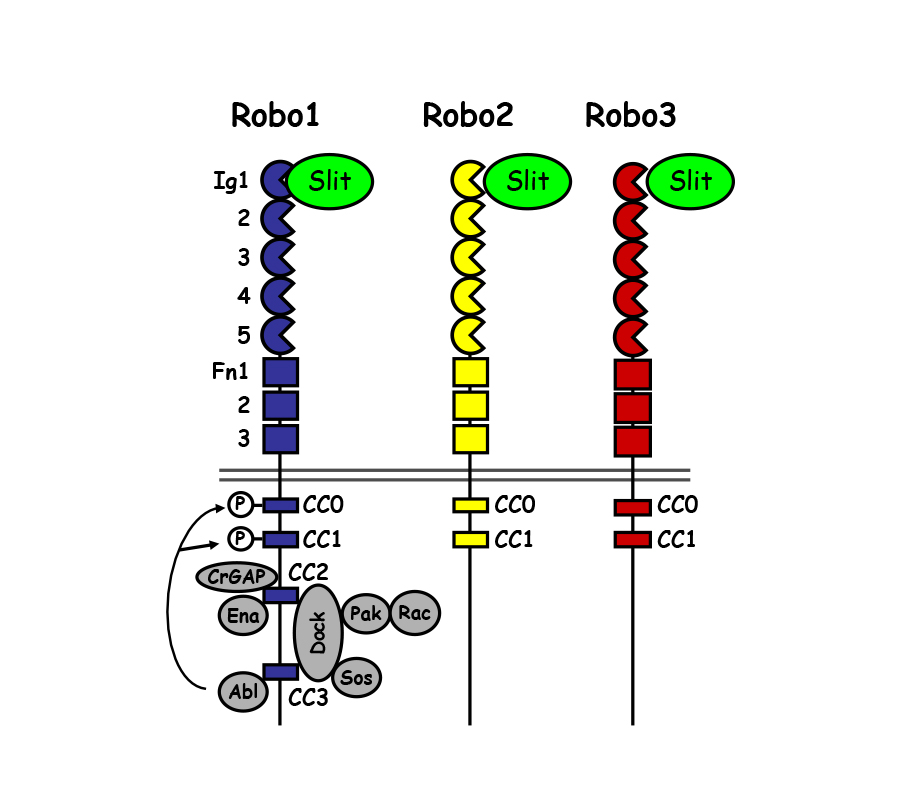
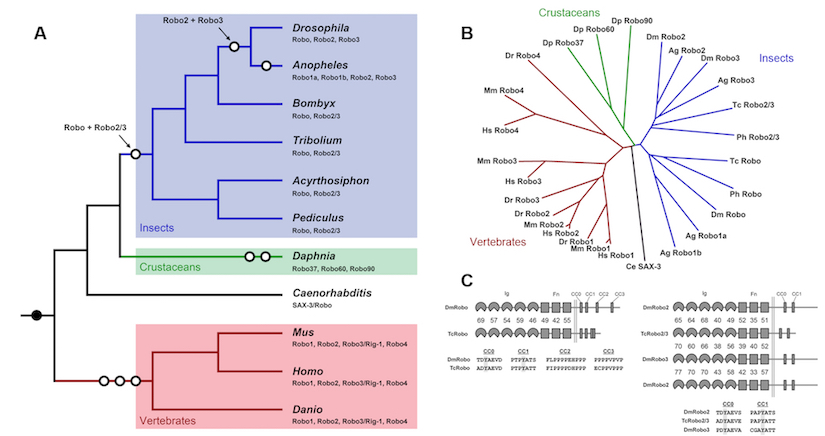
In addition to the diversification of Robo receptor function in Drosophila, it is clear that the Robo gene families themselves have diversified throughout animal evolution. Indeed, even in relatively closely related species, such as the flour beetle Tribolium Castaneum, different complements of Robo receptors are found. Interestingly in Tribolium, there are only two Robo receptors, one that resembles Drosophila Robo1 and a second Robo that shares features with both Robo2 and Robo3. Functional analysis reveals that the single Robo2/3-like receptor in the beetle can mediate both the lateral positioning and midline repulsive functions that are divided between Robo2 and Robo3 in the fly.
Close
Transcriptional Regulation of Motor Connectivity
Considerable evidence indicates that combinatorial codes of transcription factor expression function to specify motor neuron subtype identity and axon targeting in the vertebrate spinal cord, and in the Drosophila ventral nerve cord; however, little is known about how these transcriptional codes are read out at the level of specific axon guidance receptors to control path-finding and target selection. We are taking advantage of the single cell resolution that can be obtained in the Drosophila neuromuscular system to begin to bridge the gap between transcriptional regulation and specific guidance receptor expression.
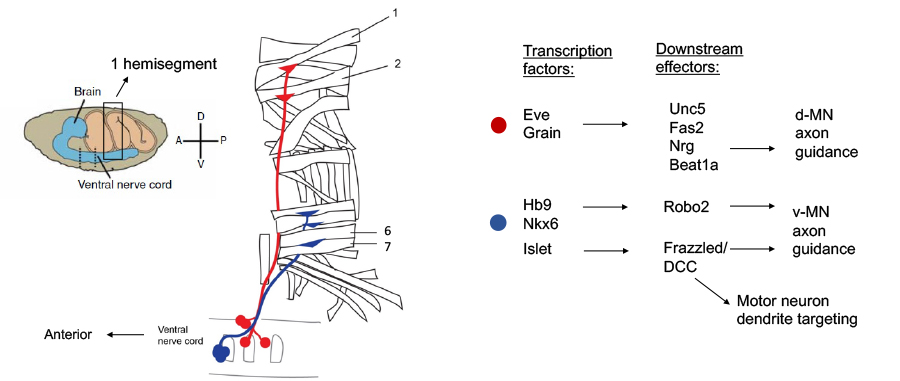
Using a combination of genetics and molecular marker analysis, we have shown that specific motor neurons exclusively express and require Unc5 for their guidance and that others express and require DCC, suggesting that the differential expression of Netrin receptors is transcriptionally regulated. Genetic and single cell mRNA expression analysis indicate that unc-5 is regulated by the even-skipped homeodomain transcription factor and that this regulation is important for correct dorsal projection of certain motor neurons.
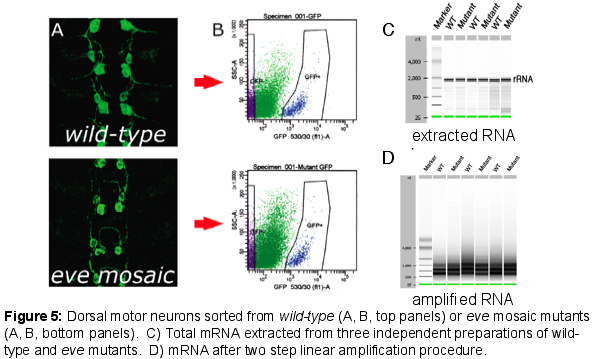
We have extended these findings by using Fluorescence Activated Cell Sorting (FACS) to purify small populations of genetically defined dorsal motor neurons from wild-type and even-skipped mosaic mutant embryos and then performing micro-array experiments with RNA from these sorted motor neurons. Using this approach, we have identified a group of cell surface molecules that are coordinately regulated by Eve. In addition, we have defined new roles for the Robo2 receptor in contributing to motor axon pathfinding and have implicated the hb9 and nkx6 transcription factors as key regulators of Robo receptor expression in motor neurons and interneurons in the CNS. More recently, we have discovered that key conserved transcription factors of the Islet family also regulate the precise targeting of motor neuron dendrites through the control Frazzled receptor expression, suggesting the possibility that the factors that dictate motor axon target selection may also control the connection specificity of motor neuron dendrites.
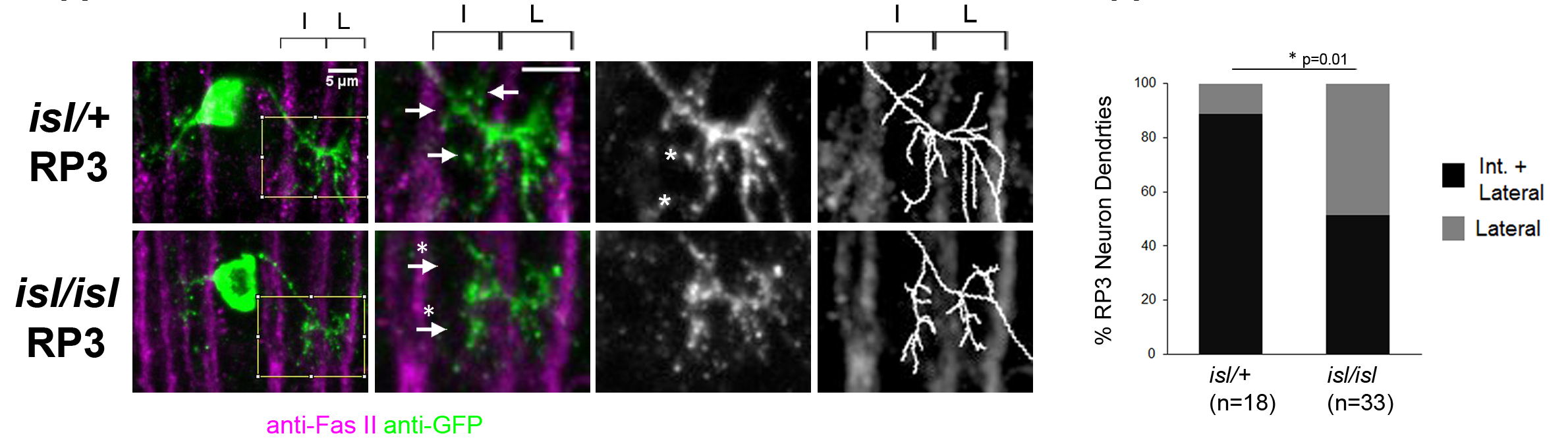
Close
Axon Guidance Pathways in Tissue Morphogenesis and Homeostasis
Since the discovery of key conserved signaling pathways that regulate various aspects of axon guidance and dendrite morphogenesis, it has become evident that these signaling pathways are reused in multiple tissue contexts during development and adulthood. In addition, increasing evidence points to critical roles for these pathways in the pathogenesis of various kinds of cancer, as well as other disease states. In order to shed further light on the mechanisms underlying the function of these important signaling pathways, we are exploring their roles in a number of non-neuronal contexts.
Axon Guidance Pathways and Drosophila Oogenesis
The well-described cell biology of the Drosophila ovary makes it a powerful genetic tool. Containing several distinctive and tractable cell populations, the ovary has emerged as a microcosm of biological inquiry: cell polarity, proliferation, and migration, as well as stem cell activity, inter- and intra-organ communication, and bacterial endosymbiosis continue to be modeled in the context of Drosophila oogenesis. Could the ovary also serve as a model to investigate the signaling axes active in developing neurons?
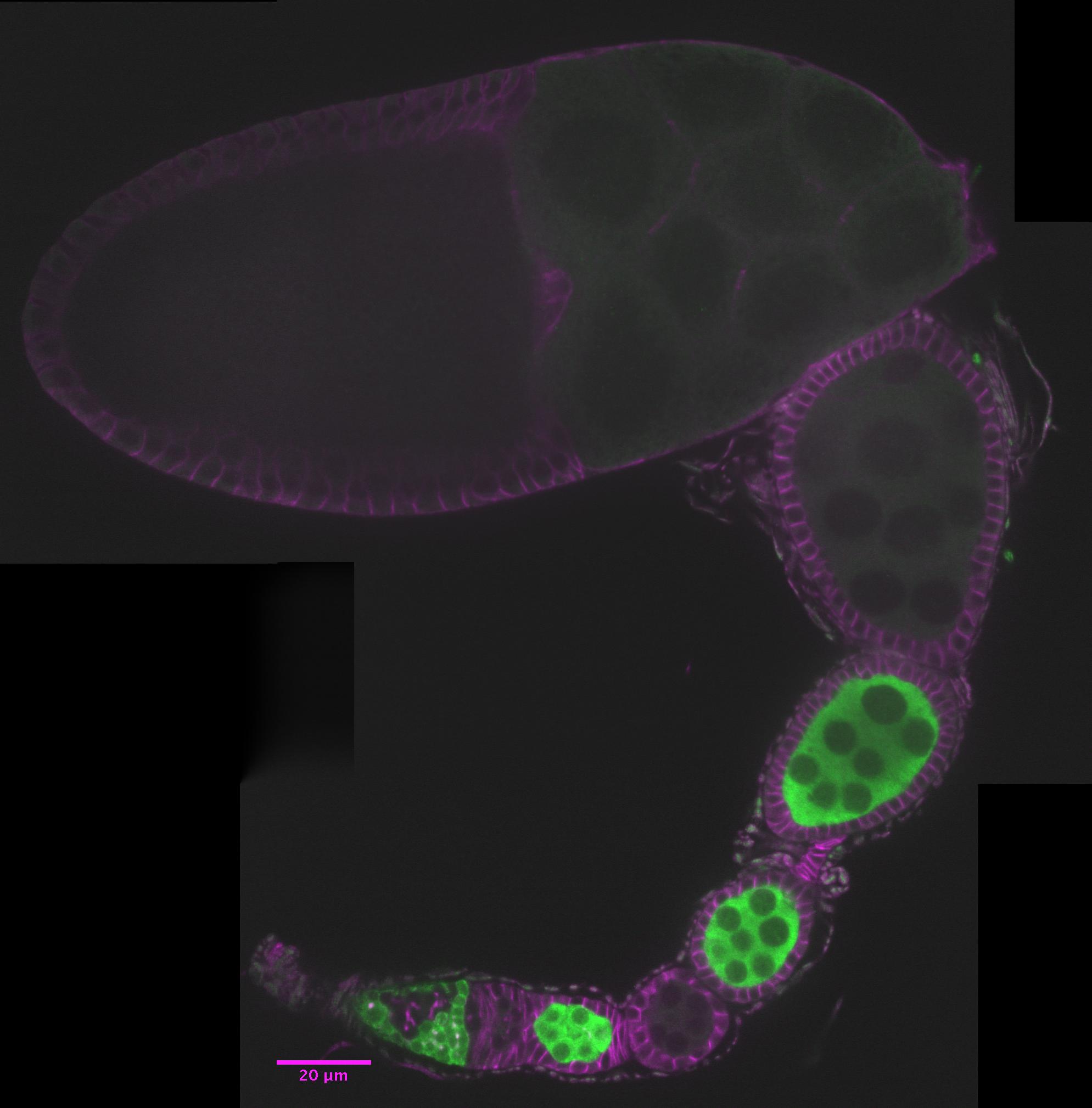
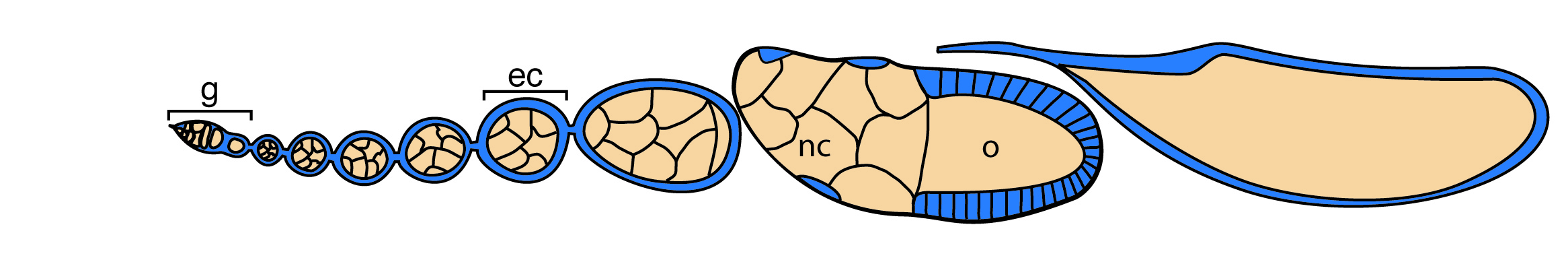
The ovary consists of 15-20 ovarioles, repeating units of continuously developing egg chambers (ec). Each egg chamber contains a germline cyst (brown) surrounded by somatic follicle cells (blue) and is produced by the two stem cell populations in the germarium (g). As it matures, the oocyte (o) takes up both yolk and the cytoplasm of its syncytial nurse cells (nc). Figure adapted from Laws and Drummond-Barbosa, 2016.
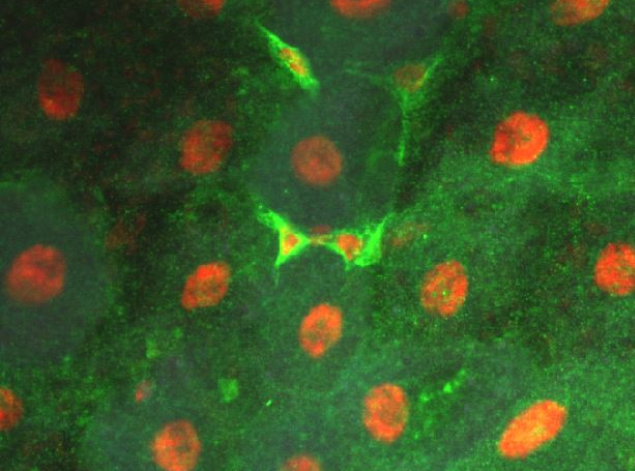
Axon Guidance Pathways and Intestinal Stem Cell Homeostasis
The adult Drosophila midgut is populated by intestinal stem cells (ISCs) which divide to replenish the ISC population, and to generate daughters fated to differentiate into either absorptive enterocytes (EC), or secretory enteroendocrine (EE) cells. The appropriate balance of these cell types is important for adult physiology and Slit and Robo2 have been shown to contribute to tissue homeostasis in this process through negatively regulating the proportion of EE cells in the tissue (Biteau 2014, Bardin 2017, Nagy 2017). We are interested how these classic axon guidance molecules function in the context of stem cell lineage commitment. Do Slits and Robos regulate these processes through similar signaling mechanisms to the mechanisms that they use to guide axons? Or do these functions represent distinct receptor signaling activities? Given the diverse roles of guidance molecules in other tissue contexts, it is important to define the ways in which they impinge on what appear to be quite distinct biological events.
Close
