Penn Clinical Research (CR) Onboarding Program
The Penn Clinical Research (CR) Onboarding Program will provide comprehensive training in accordance with Good Clinical Practices (GCP) for investigator-initiated, industry-sponsored and grant-funded clinical research. It will present best standards of practice and regulatory requirements for critical job functions of the Clinical Research Coordinator and methods for achieving these standards and requirements.
University policy (SOP401) mandates that any staff member performing function(s) related to human subjects research for which the IRB would require CITI and/or HIPAA training are required to complete the Penn CR: Onboarding.
NOTE: OCR’s clinical research training content provider ACRP (Association of Clinical Research Professionals) is now on Moodle Workplace. Please see this Guide on how to navigate this change once you find a course of choice in Workday@Penn Learning system.
Steps for Completing Penn Clinical Research Onboarding Program
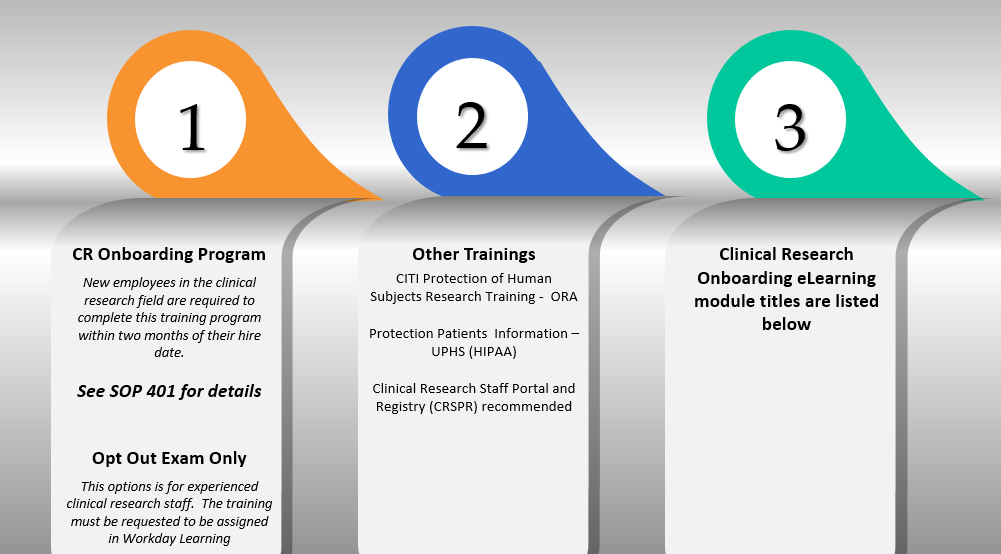
- You can self-assign the Penn Clinical Research (CR) Onboarding via Workday Learning
-
The Penn Clinical Research: Onboarding - Opt Out Exam must be assigned by OCR. Send the request via psom-ocrops@box.upenn.edu.
-
Completion of CITI Protection of Human Subjects Research Training - ORA
- Completion of Protecting Patient Information (HIPAA) (UPHS)
- Registration in the Clinical Research Staff Portal and Registry (CRSPR)
Course content in Penn CR Onboarding Program
- Ethics and Human Subject Protection: A Comprehensive Introduction
- Good Clinical Practice (GCP)
- Institutional Review Board (IRB) 101
- Clinical Trial Finance Basics
- Informed Consent
- Recruitment
- Study Start-up and Management
- Study Close-out overview
- Full Onboarding Exam
Note: When submitting external training from another institution you will need to provide the original completion certificate and a detailed description of content covered in the course. Certificates must have date of completion listed in order to be used to satisfy training. For example: you could use any one of the following options to complete or renew GCP Training. Send an email with the completion certificate to the Office of Clinical Research Operations team
- Good Clinical Practice (GCP) Simulation* training provided in Penn's Workday through Association of Clinical Research Professionals (ACRP)
- CITI’s GCP – Social and Behavioral Research* training provided through CITIs website
- CITI’s Good Clinical Practice (GCP)* training provided through CITIs website
*these trainings are identified by TransCelerate BioPharma as necessary to enable mutual recognition of GCP training among trial sponsors.
1. Who should take the Penn CR: Full Onboarding Program?
All Clinical Research (CR) staff are required to complete the Penn Clinical Research Onboarding Program in two months of hire or being assigned via Workday Learning in "Career".
2. How long does it take to complete the program?
The program takes approximately 12 hours to complete. The program is required to be completed within two months of hire date. There are no printed materials, and the course is entirely online via Workday Learning and ACRP.
3. Do I have to complete the Penn CR Onboarding Program?
If you are engaged in human subjects research (patient and/or PHI), you must complete all of the listed onboarding e-learning modules in the program. To now whether your human subjects research study meets the NIH definition of a clinical trial? Click here to find out
4. What courses are covered in Penn CR Onboarding Program?
- Good Clinical Practice (GCP) Simulation
- Ethics and Human Subject Protection: A Comprehensive Introduction
- IRB 101
- Clinical Trial Finance Basics
- Informed Consent Simulation
- Recruitment
- Study Start-up and Management
- Study Close-Out Overview
- Clinical Research Technology
- Onboarding Exam
5. What are the benefits of taking the eLearning modules OCR provides?
- Quick access to relevant education and training material
- Personalized training schemes tailored to the learner’s objectives
- eLearning modules will assist you in becoming more proficient in your research role
Is this the same as the certifications offered by SOCRA and ACRP?
No, the Penn CR Onboarding is not recognized nationally - only at Penn. The purpose of this certification is to provide the coordinators at Penn with a centralized training program in an effort to improve the quality of research, efficiency of conducting research, and the safety of human subjects at Penn. Penn CR Certification and re-certification training program will no longer be available/ will be sunset starting October 30, 2023.
Continue Education Units
Credits from completed trainings will remain in Workday Learning. These could be used for CEUs with external certification providers such as ACRP, SOCRA. Certificates can be printed from your career or transcript.