Research
The wet blanket theory postulates that anesthetic drugs work non-specifically, binding promiscuously at the molecular level and globally at the neuronal (and glial) level to envelope the entire brain and comprehensively perturb brain function to enhance inhibitory signaling and inhibit excitatory signaling and consequently yield states of unconsciousness. However, alternative theories highlighting shared traits among the hypnotic states common to both NREM sleep and sub-surgical levels of many anesthetic drugs suggest that a component of the hypnotic state may arise through specific targeted actions of anesthetics upon the endogenous neural circuits that generate natural sleep.
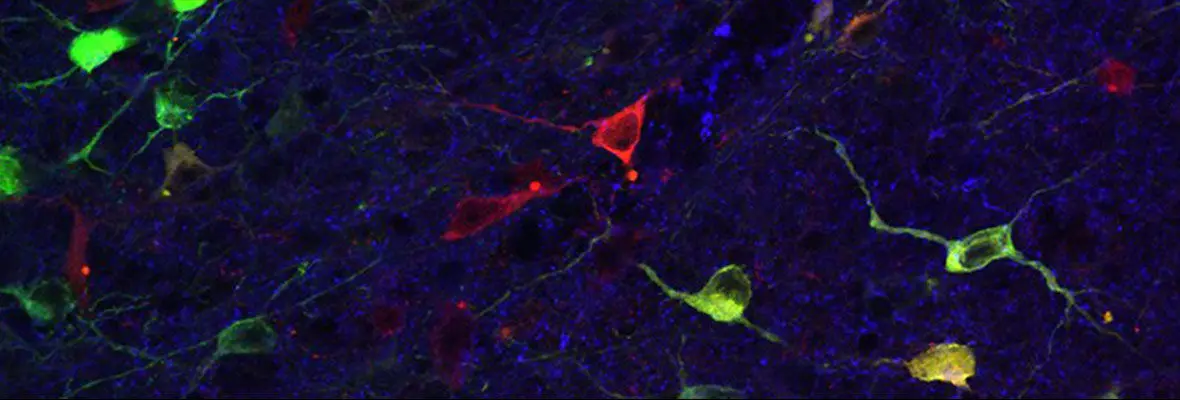
Some of the most important clues for site-specific actions of anesthetic drugs have been provided by the clinical observation that a subset of patients suffering from narcolepsy (a primary neurological disorder in the organization of sleep and wakefulness) have problems exiting states of anesthesia. As narcolepsy is a disease that arises from a loss of orexin/hypocretin neurons confined to the hypothalamus, this finding which the Kelz lab replicated in mice, suggests that general anesthetics can and do have specific interactions with discrete populations of neurons in the CNS. Work in the Kelz lab uses combinations of molecular genetics, histochemistry, circuit mapping, electrophysiology, and behavioral assessments to phenotype the anesthetic state in multiple ways.
As drugs that classically enhance inhibitory signaling and inhibit excitatory signaling, general anesthetics are not predicted to directly depolarize neurons in the CNS. However, work in the Kelz lab has found that of all the neurons that could be depolarized and increase their firing in response to anesthetics exposure, discrete populations of putative-sleep promoting neurons are indeed activated by general anesthetic drugs.
In order to localize and characterize the important features of inhaled anesthetic protein targets, we, in collaboration with the Eckenhoff lab, have found anesthetic-like activity of recently developed novel anesthetic photolabels in a variety of in vitro systems and the tadpole. Our lab is translating these findings to rodents to better understand anesthetic binding and actions in the brain and anesthetic sensitivity in mammals. This project will provide the foundation for a novel mechanism of drug action, which will ultimately lead to better anesthetic management in patients and new and better anesthetic drugs for patients.
Together with our collaborators and other investigators in the Neurobiology of Unconsciousness group, the Kelz lab conducts volunteer studies in humans designed to reveal insights into the ways in which the human brain enters and exits states of general anesthesia, to determine how the CNS “reboots” after leaving the abyss of general anesthesia, and how signatures of the anesthetic state as measured both through high density EEG and ECoG may help to reveal whether patients are adequately anesthetized to block conscious perception and new memory formation.