NIH Research (R) Grants
NIH Eligibility:
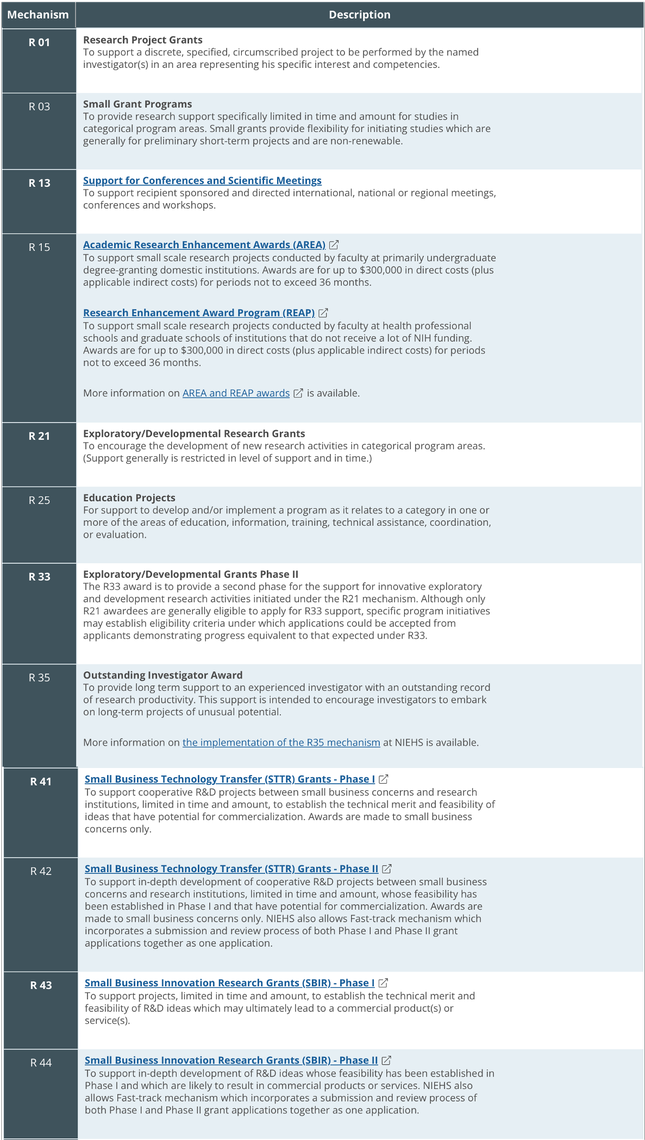
These awards are made to institutions in support of a wide variety of research-related programs. Eligibility can vary depending on the NIH center so it is crucial that you ask your PI for the link to the specific FOA to which they are applying.
PSOM Eligibility:
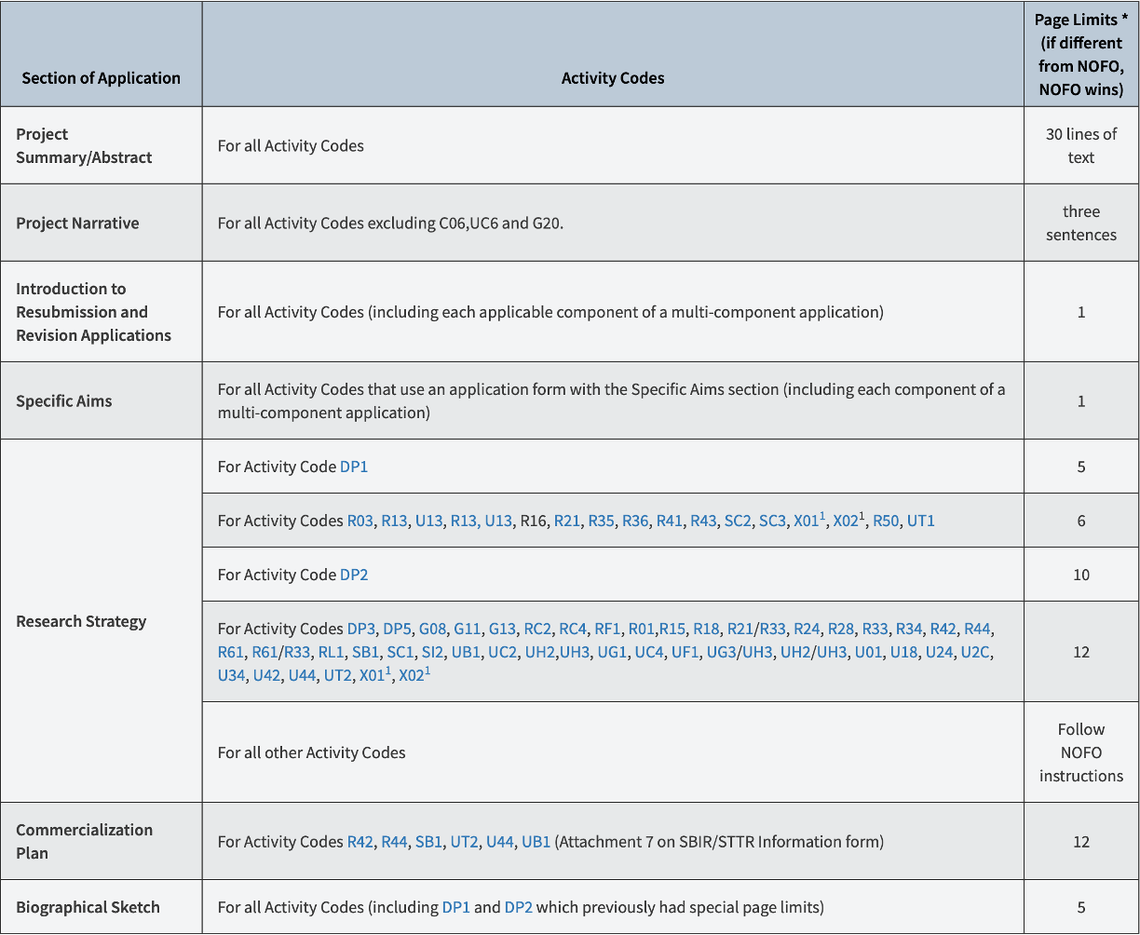
- All proposals submitted to sponsors for external support must carry as principal or co-investigator at least one person in a professorial track holding the academic rank of professor, associate professor, assistant professor, or be appointed as a clinician educator. A principal investigator or co-principal investigator is an individual designated by the University and approved by the sponsor to direct a project funded by an external sponsor. S/he is responsible and accountable to the University and sponsor for the proper programmatic, scientific, or technical conduct of the project and its financial management.
- The principal investigator must be an employee of the University or hold an adjunct or emeritus appointment.
- Individuals who are trainees, whether or not they are also employees (such as postdoctoral fellows/associates, students, interns or residents), may apply for external sponsorship only with the approval of a faculty sponsor or mentor as indicated either on the application or the ORS Transmittal and Approval form.
- Exempt staff wishing to apply for sponsored projects must seek the approval of their department head, the appropriate division Vice President or Dean, and the Vice Provost for Research or his designee (Executive Director, ORS).
- All applications for external sponsorship must indicate the approval of the appropriate department chair and dean, indicating the availability of resources necessary to carry out the project.
- Individuals not meeting the above criteria may, by demonstrating sufficient cause, petition the Executive Director of ORS for approval to submit an application to an external sponsor. Such approval will usually require the agreement of the Vice Provost for Research.
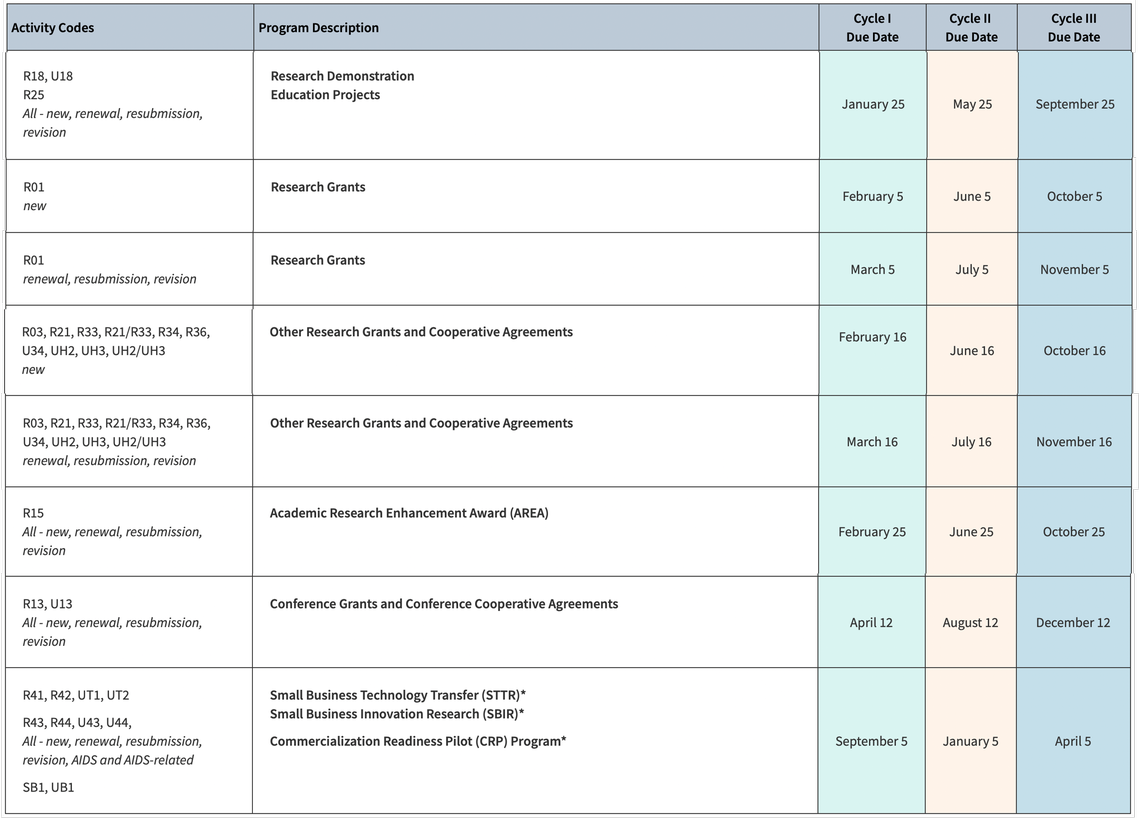
Budget limits can vary depending on the NIH center so it is crucial that you ask your PI for the link to the specific FOA to which they are applying. As per our legally binding F&A agreement with the Department of Health and Human Services, an F&A rate of 62.5% must be applied to all R grants.
To initiate the R grant submission process, you must perform two steps:
- Build and assemble the record in PennERA
- Submit for pre-review.
R grants are first reviewed by an ORSS reviewer whose primary focus is checking to ensure that eligibility, budgeting, salary, and effort conform to PSOM policies. Once your ORSS reviewer has reviewed the record, they will send you an email to let you know if they have approved the record and what changes they recommend.
To complete the submission process, you must then:
1. Finalize the record
2. Submit it for final review so that an ORS reviewer can give final University approval and submit the proposal to NIH.
R grants are then reviewed by an ORS reviewer whose is checking to ensure that all proposal documents conform to University policies and funder requirements. Once your ORS reviewer has reviewed the record, they will send you an email to let you know if any changes need to be made before they can approve it or if it has been submitted.
All proposals must be submitted to ORSS five business days before the deadline. If you do not submit within the required timeframe, neither ORSS nor ORS can guarantee an adequate review.
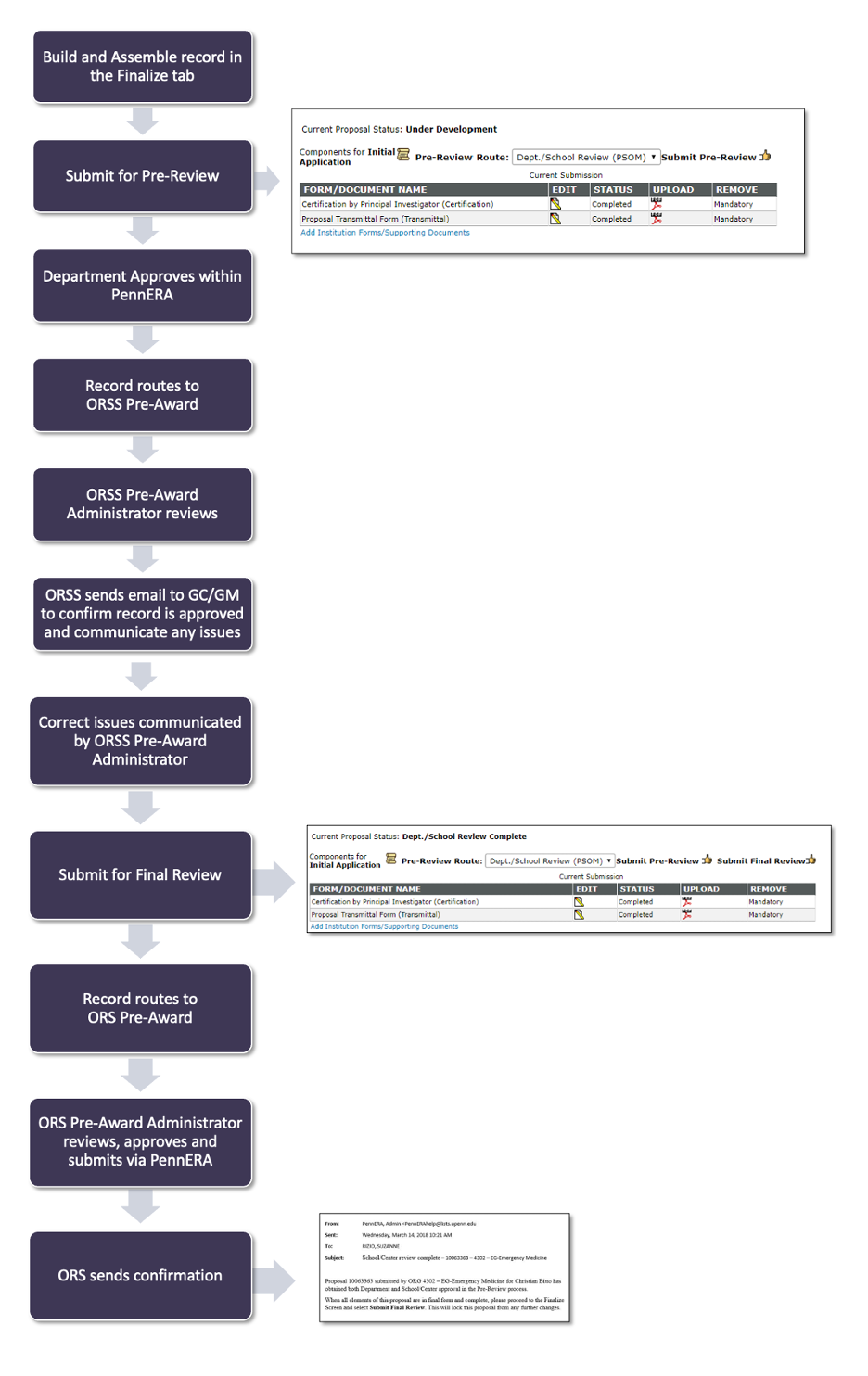 Note: If your department has obtained SO Authority, your department SO will review and submit the record.
Note: If your department has obtained SO Authority, your department SO will review and submit the record.