Cultured Axonal Injury
Diffuse axonal injury (DAI) is thought to be the most common and important pathology in mild, moderate, and severe traumatic brain injury. In severe cases of DAI, shearing forces can cause primary disconnection of axons.
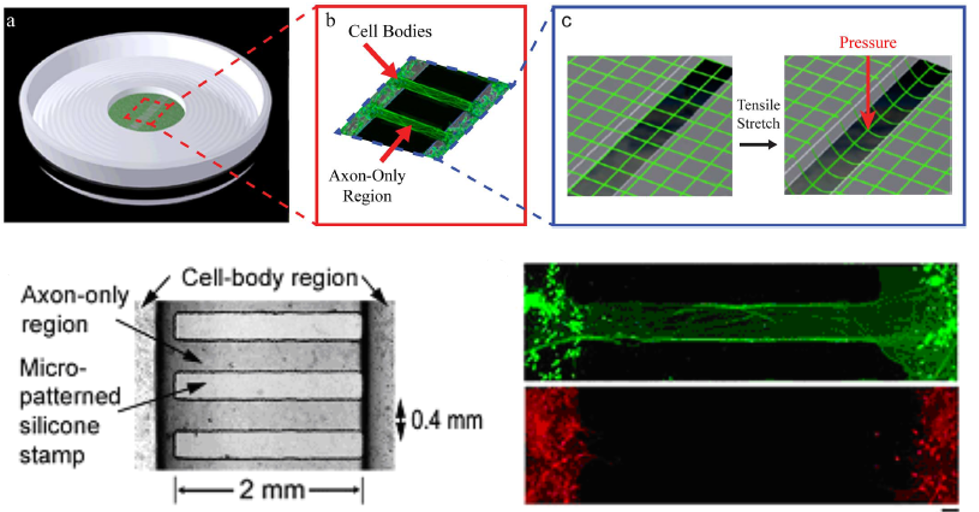
However, the vast majority of posttraumatic axonal pathologies evolve over time due to a series of deleterious cascades that include activation of proteases, second messengers, and mitochondrial failure. Using a cultured axonal injury (CAI) model we have demonstrated that dynamic mechanical stretch injury of cultured axons replicates many of the morphological and ultrastructural changes found in DAI in vivo.
With this model:
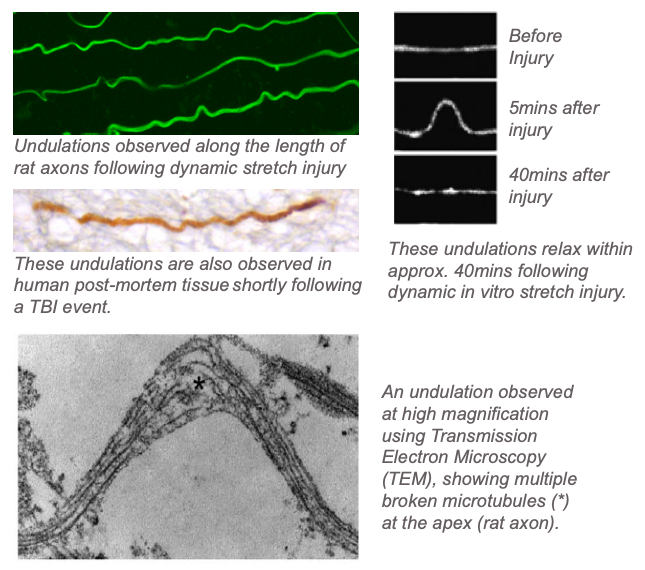
- We have shown that immediately following dynamic stretch injury, axons show an undulated morphology, which eventually relaxes to its original shape within approximately 40mins. When examined at the ultrastructural level using Transmission Electron Microscopy (TEM) techniques, we observe that these undulations are due to the breaking of microtubules. [Smith et al, JNeuroscince, 1999] [Tang-Schomer et al, FASEB, 2010][Tang-Schomer et al, Exp Nerology, 2012].
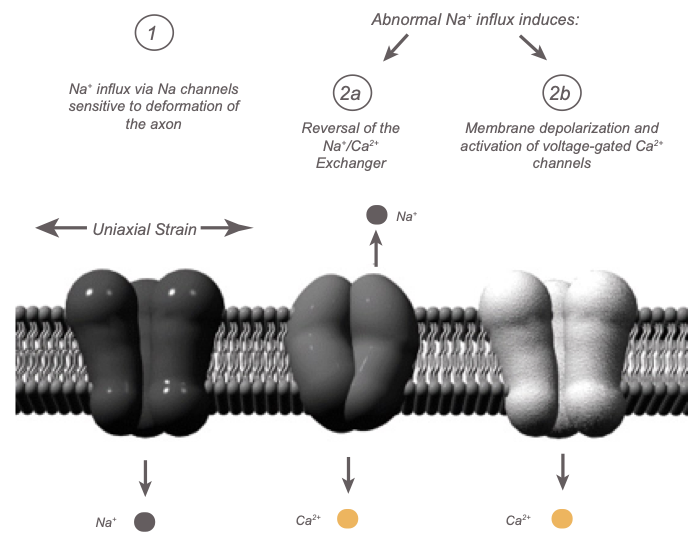
- We found the first evidence that rapid stretch of axons induces an immediate increase in intra-axonal calcium levels ([Ca2+]i) and that this response could be completely reversed with the voltage-gated sodium channel (NaCh) blocker tetrodotoxin (TTX).
Thus, while sustained elevated [Ca2+]i may be an important mediator of secondary damage to axons after trauma, as has been previously proposed, this increase in [Ca2+]i is dependent on trauma-induced Na+ influx through NaChs. However, the disposition of NaChs following dynamic stretch injury has not previously been examined.
Non-inactivation of NaChs has been shown to cause pathological Na+ influx and membrane depolarization, a state that could potentiate Ca2+ influx through voltage-gated Ca2+ channels and reversal of the Na+/Ca2+ exchanger. Most recently, researchers have used the model of dynamic stretch injury of axons from primary cortical neurons to find that Na+ influx through NaChs due to axonal deformation triggers initial increases in [Ca2+]i and subsequent proteolysis of the III-IV intra-axonal loop of the NaCh alpha-subunit, suggesting a unique "feed forward" deleterious process initiated by mechanical trauma of axons. [Wolf et al, JNeuroscinces, 2001] [Iwata et al, JNeuroscience, 2004] [von Reyn, JNeuroscience, 2009]
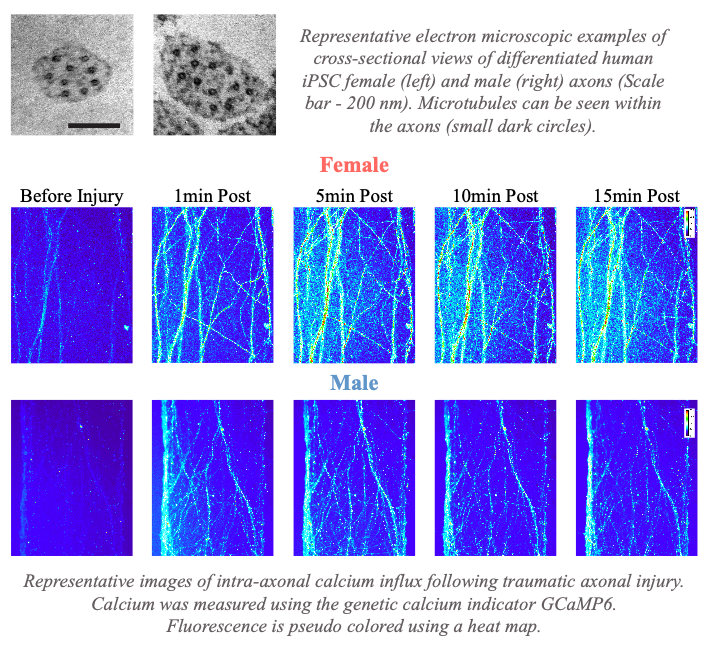
- We recently reported that female axons are, on average, smaller in diameter with a more simplified microtubule geometry compared to male axons. This sex difference in axonal ultrastructural results in female axons having greater microtubule damage and ionic disruption due to mechanical trauma than male axons, leading to a greater extent of axon degeneration in vitro. Notably, this may reveal one mechanism to explain why female athletes participating in the same sport as males appear to have a higher rate of concussion symptoms and worse outcomes. [Dollé et al, Exp Neurology, 2018]