Cover Images & Graphical Abstracts

(B-ALL), but loss of the CD19 epitope has been implicated in tumor relapse. Sotillo and colleagues compared paired CD19-positive,
pre–CART-19 and CD19-negative, post–CART-19 relapsed pediatric B-ALL samples and found hemizygous deletion of CD19 and mutations affecting CD19 exon 2 in a subset of relapsed tumors. Alternatively spliced CD19
transcripts were also specifically identified in relapsed samples, including a splice variant with exon 2 skipping (CD19 Δex2) that resulted in expression of a functional truncated protein. CD19 Δex2 expression provided a proliferative advantage and partially rescued the effects of CD19 loss. In addition, CD19 Δex2–expressing cells remained viable upon CART-19 exposure, suggesting that alternative splicing can lead to epitope loss and evasion from CAR T-cell therapy. For details, please see the article by Sotillo and colleagues on page 1282.


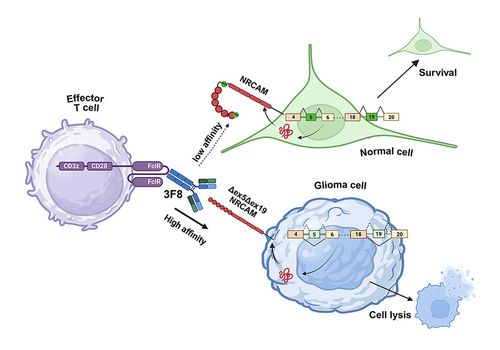
• The hallmark of pediatric high-grade gliomas is pervasive mis-splicing of microexons
• Transcripts encoding the neuronal cell adhesion molecule (NRCAM) skip exons 5 and 19
• Δex5Δex19, but not full-length, NRCAM drives tumor growth in orthotopic glioma models
• Δex5Δex19 NRCAM-selective mAb enables killing by T cells expressing the Fc receptor

