Contract Negotiation
Contracting Offices at Penn
There are several contracting offices at Penn that are responsible for negotiating research agreements, which include: (i) Office of Research Services (ORS), (ii) Abramson Cancer Center (ACC) Legal Affairs, (iii) OCR Legal, and (iv) Penn Center for Innovation (PCI).
Responsibility is divided among the contracting offices based on (i) the category of the outside party that Penn is contracting with for the research project (i.e. for-profit/industry partner vs. government agency/not-for-profit partner), and (ii) whether the research involves human subjects required to give CURRENT informed consent and therefore requires IRB oversight.
-
- Office of Research Services (ORS) negotiates and manages research agreements with federal government agencies and not-for-profit research partners and funders, which may or may not involve human participants.
- Abramson Cancer Center (ACC) Legal Affairs negotiates cancer-related clinical research agreements and related enabling agreements (e.g. CDA, NDA, DUA and MTAs) with for-profit/industry partners for research involving human subjects who are required to give current informed consent and that requires IRB oversight.
- OCR Legal negotiates non-ACC clinical research agreements and related enabling agreements (CDA, NDA, DUA and MTAs) with for-profit/industry partners for research involving human subjects who are required to give current informed consent and that requires IRB oversight.
- Penn Center for Innovation (PCI) negotiates agreements with for-profit/industry partners for research that does not require IRB oversight (e.g. basic and translational research, which could involve animals, human cadavers, and human biobank samples where no new informed consent is required).
When research projects receive funding from multiple sources (federal, not-for-profit and for-profit/industry sources), and/or involve both basic science and clinical research activities involving human subjects, the contracting offices will confer to decide which is the most appropriate office to negotiate the research agreement(s). That decision is then communicated to the investigator.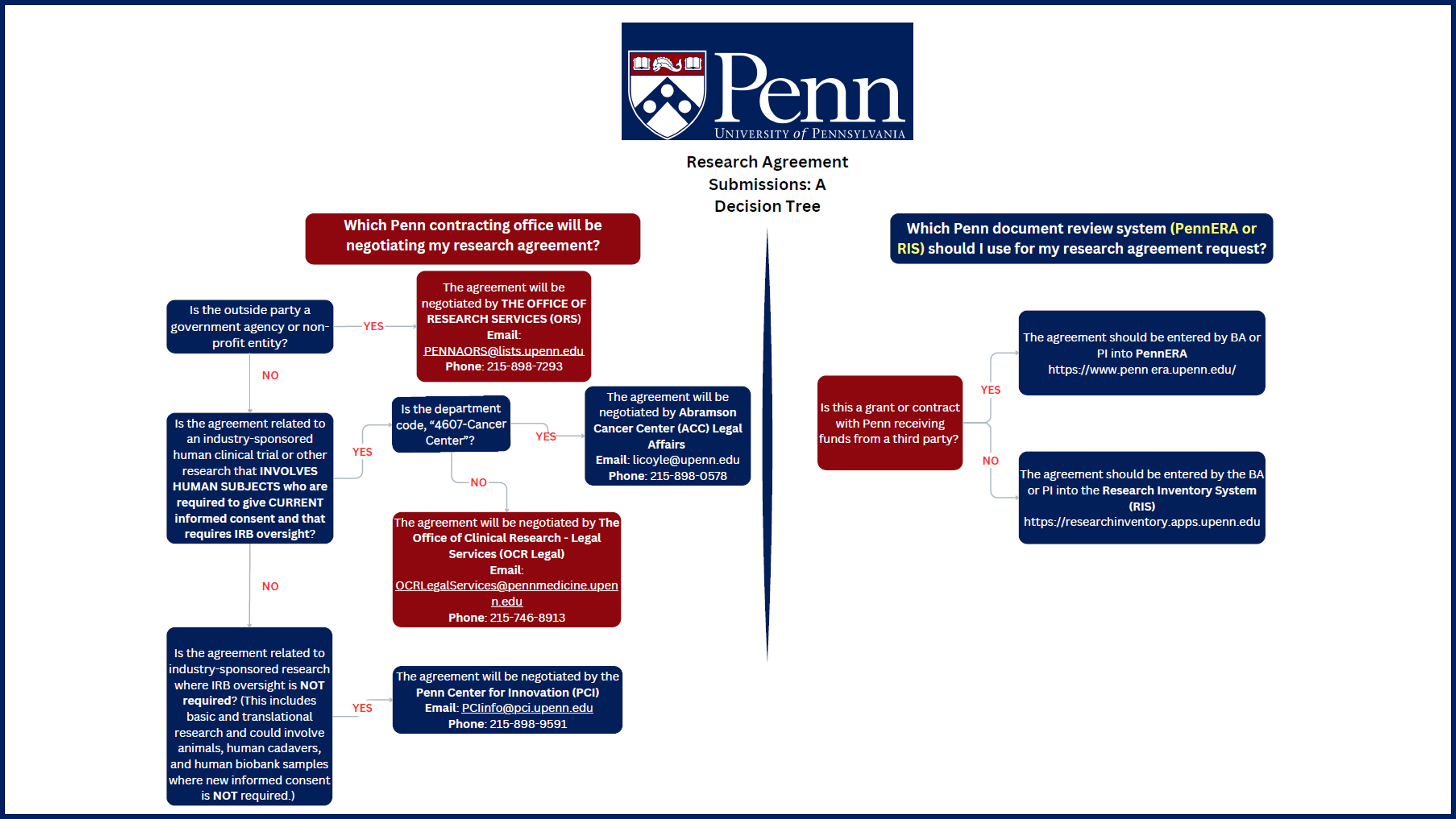
Types of Agreements
CTAs are contracts that facilitate the funding of clinical trials at Penn by third parties, and establish the rights and obligations of the parties involved in the conduct of a clinical trial at Penn (i.e. Penn, Industry partner, and a CRO, if applicable) including confidentiality obligations, rights to the results and intellectual property that are generated in the performance of the trial, the right to publish the results, use of name, and the allocation of risks associated with performance of the trial. CTAs are necessary to enable Penn’s conduct of clinical trials, which could be either industry-initiated OR Penn investigator-initiated.
When Penn and an industry partner anticipate that Penn will perform multiple clinical trials with an industry partner, they may decide to enter into a Master CTA. The Master CTA delineates in one agreement the generally relevant terms and conditions that will apply to future clinical trials performed under the Master CTA. For each new clinical trial under the Master CTA the parties will execute a simple new agreement – sometimes called a “Work Order” or an “Addendum” – that incorporates the terms and conditions agreed upon in the Master CTA, and adds trial-specific terms, including any modifications to the terms and conditions of the Master CTA applicable for that particular trial, and includes the applicable budget and payment terms. Having a Master CTA in place with an industry partner can substantially expedite the agreement negotiation and execution process for clinical trials falling under the Master CTA.
SRAs are contracts that facilitate the funding of defined research projects at Penn, which are not clinical trials, by industry partners, and define the rights and obligations of each party to the agreement, including confidentiality obligations, rights to the results and intellectual property that are generated in performance of the project, the right to publish the results, use of name and the allocation of risks associated with performance of the research project.
CRAs are contracts between Penn and one or more industry partners that are cooperating and jointly participating in the conduct of a research program. A CRA defines the role and obligations of each party participating in the collaborative research effort and defines the rights and obligations of each party to the agreement, including confidentiality obligations, rights to the results and intellectual property that are generated in performance of the project, the right to publish the results, and use of name and the allocation of risks associated with performance of the research program.
CRAs are similar to Sponsored Research Agreements but govern more varied collaborative research endeavors. A CRA would be appropriate, for example, where the project involves participation in the research by scientists from both Penn and the industry partner, or where the industry partner is also providing materials for use in the research. Projects giving rise to a CRA may or may not involve funding from the industry partner.
CDAs govern the exchange of confidential information between two or more parties and are necessary to protect the parties’ valuable confidential and proprietary information from disclosure to third parties and from unauthorized uses. CDAs allow the parties to exchange defined categories of confidential information to further a clearly defined and limited purpose (e.g. review of a protocol and other materials to evaluate Penn’s potential participation a clinical trial) and outline what the receiving party can and cannot do with the disclosing party’s confidential information.
Service Agreements are contracts that Penn enters into to engage outside parties to perform well-defined work such as routine testing, standardized procedures, or other services on a pay-for-service service. In a Service Agreement, funds flow out of Penn to pay the outside party for performance of the defined service(s).
From time to time an industry partner will propose contracting with and paying Penn to perform services on the industry partner’s behalf, as opposed to funding Penn’s performance of exploratory research. This type of arrangement, where Penn is paid to perform a service, requires a Fee-for-Service Agreement. Given Penn’s status as a non-profit institution, research, teaching and training activities conducted at Penn must be beneficial to the general public, advance Penn’s research and education mission and not be for the sole benefit of a commercial party. As a result, Penn only utilizes Fee-for-Service Agreements in circumstances where the work to be conducted is consistent with Penn’s academic and research missions, is not exploratory in nature, and does not involve intellectual contributions by a Penn researcher.
MTAs are contracts that govern the transfer and use of research materials (e.g. biological materials, study drug or device, equipment that will be used in a defined research program) between organizations. An MTA is required where Penn or one of its researchers wishes to receive research materials from a third party (e.g. an industry partner, a federal agency, or another university) to facilitate research, or conversely when a research partner wishes to receive research materials from Penn for a defined research program. The MTA establishes how the receiving party can use the providing party’s material and defines the parties’ rights to the results and intellectual property that are generated using the research materials.
DUAs are contracts that govern the transfer and use of data between organizations. A DUA is required where Penn or one of its researchers wishes to receive and use data from a third party (e.g. an industry partner, a federal agency, or another university) to facilitate research, or conversely when a third party wishes to receive data from Penn. The Data Use Agreement outlines how the receiving party can use the providing party’s data and defines the parties’ rights to the results and intellectual property that are generated using the transferred data.
An LDS is a data set containing protected health information (PHI) in which certain direct identifiers specified in the Privacy Rule, have been removed, but that still contains PHI. A DUA for an LDS is a contract that is required under The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule anytime Penn discloses an LDS to a third party. The DUA for an LDS is a legally required data transfer mechanism that facilitates the transfer of the LDS from Penn – a “covered entity” as defined under HIPAA – to a third party for certain limited purposes. Under the Privacy Rule, an LDS may be disclosed to an outside party without a patient’s authorization if the purpose of the disclosure is for research, public health, or health care operations purposes and the person or entity receiving the information signs a DUA for an LDS with the covered entity or its business associate.
Manufacturing Agreements or Development Agreements are agreements that govern the manufacture of a product, or development activities relating to product manufacture. These agreements delineate the scope of work and establish terms and condition including ownership of intellectual property, and warranty and indemnity provisions, which allocate risks associated with the manufacturing process.
Under the terms of a Manufacturing Agreement, the party manufacturing the product may be required to enter into a separate Quality Agreement The Quality Agreement ensures that any research and development activities involving manufacturing work comply with all legal requirements, any agreed-to specifications and also to ensure an understanding between the Parties regarding their respective responsibilities regarding quality assurance.
A subaward agreement is needed when a portion of funding received for a sponsored research project (including clinical trials) is passed through to another entity (the “Subawardee”), which performs a portion of the sponsored project's scope of work. For example, Penn may enter into a subaward agreement with another university performing a clinical trial under a Penn-Investigator Initiated protocol and where the study is being funded by third party (i.e. federal agency or industry partner). The Subawardee is responsible for adhering to the terms and conditions of the subaward agreement including those flowed down from the agreement between the third party funding the Study and the prime recipient of those funds.
Frequently Asked Question
Anytime Penn is partnering with a person or entity outside of Penn, a legally binding agreement is needed. The agreement is necessary to establish the scope of the research relationship, the expectations and obligations of the parties, and to minimize risks to Penn associated with the research relationship. Agreements facilitate the funding of research, the transfer of materials, including biological samples, the transfer of data, collaboration between two or more parties, commercial development of a Penn product, device, or intervention, etc. Once negotiations are finalized an agreement must be signed by a Penn authorized signatory to be legally valid and binding. Each contracting office has authorized signatories on staff.
- Industry funded projects- Requests for research agreements where Penn is receiving funds from an industry partner should be submitted in PennEra and any supporting documents (e.g. the protocol, ICF, research agreement template provided by the third party) should be uploaded to the PennEra record. Simultaneously, a notice should be sent to the OCR Legal mailbox with a request for parallel review {termed “parallel review” because the agreement and budget are meant to be negotiated in parallel}. You will receive a notice from the negotiator assigned to the agreement. *For expediency, you are advised to simultaneously finalize your budget, submit the study materials to the IRB, and, if applicable, any conflict of interest, for their respective reviews.
- Requests for Industry partnered, non-monetary, agreements (e.g. DUAs, MTAs, CDAs), accompanied by all supporting documents, should be uploaded into the Research Inventory System.
A full list of Penn’s master agreements can be found in this document.
Yes. Costs associated with Vendormate must be accounted for in the budget section of industry sponsored clinical trial agreements. Vendormate is a 3rd party vendor Penn Medicine uses to perform background checks on pharmaceutical company representatives, including their immunization status. These background checks help Penn protect patients, especially immunocompromised patients, in clinic spaces. Registration in Vendormate costs $250 per year. Penn requires yearly renewal by the company, and updates of the representatives’ immunization records. The cost for this is included in the budget template by OCR Finance (search for Prospective Reimbursement Analysis in Budget category) so teams can incorporate it before they negotiate with the pharmaceutical sponsor. The pharmaceutical representative category is also extended to CRO/ sponsor monitors that will be in clinic spaces.
- The link below is a brief step by step tutorial showing how a Pharmaceutical company representative registers on the platform. The screen shot below shows the main website, and video tutorial that the vendor can access at: https://registersupplier.ghx.com/reg/network/vendor/
- Vendormate Policy at HUP
Amendments to corporate-funded clinical trial and clinical research agreements may be initiated by creating a child record in the PennERA record of the original agreement (with the exception of “no-cost extensions”).
A no-cost extension is an amendment that (i) solely requests no-cost end date extensions, (ii) has no contractual language changes, and (iii) has a specific end date listed in the contract. If you want to request a no cost extension, to an existing corporate-funded clinical trial or clinical research agreement, please send an email to OCR Legal Services.