Patient Recruitment Management System (PRMS)
Features
iConnect
About
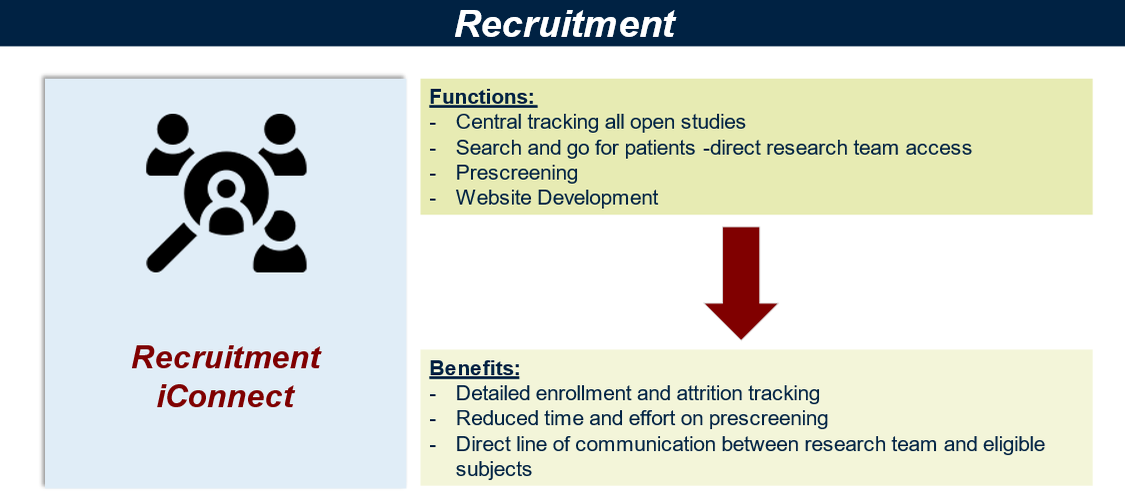
iConnect Penn Medicine's clinical trial listing, search, and recruitment platform. It is a key component of research at Penn for both research teams and research subjects. From the potential subject perspective it presents and array of easily searchable trials and a way to connect with research teams. It is also the central research volunteer registry.
For research teams it provides a clinical recruitment portal where subjects can be pre-screened, enrollment tracking, communication, and outreach to potential participants in the volunteer registry. Advanced functions include mobile app development for clinical research, bi-directional texting, ePRO data collection, analysis and more.
Clinical Research Teams
As of October 2019, all clinical trials will be made viewable and searchable to patients and the public. Penn Medicine has an obligation to our patients to increase access and visibility to all clinical research being conducted on premises.
Watch a video on iConnect. iConnect at Penn Medicine is powered by TrialX.
Training
To list your trial and start using iConnect complete the 30 minute Workday Learning e-module “iConnect e-module Training”. We recommend logging into WD before clicking this link.
Please be sure to complete the test at the end of the module and fill the access request form at the end of the training (this will be a REDCap form). Click the submit button after the training to request access. The OCR team will create an account for you within 24-48 hours.
Once we build your account, we will be able to link your profile to your study in iConnect. Alternately, you should also be able to add your study to iConnect if it doesn’t already exist in the system.
To add a new research team member to a study, send a request to OCR (psom-ocr@pobox.upenn.edu), with the Subject: iConnect- Addition or Removal of study team member.
Features
- Using Pennkey to login: iConnect Login is now a Single Sign On using Pennkey and password
- IRB-approved Studies Imported by API: research studies are imported into iConnect from the data warehouse post IRB approval, reducing manual entry of information. Studies are initially listed as recruiting. The PI and two study team members will receive a system-generated email to their Outlook email alerting them that the study is active on iConnect.
- Clinical Trial Listing: allows potential participants to conduct quicker web search and directly message the study team.
- Reporting Dashboard: At a glance metrics of trial clicks, views, and patient referrals
- Pre-screening Tool*: Create short pre-screening questions into your iConnect trial page, reduce false positives before you see patient on-site.
- Muliti-Study Locations: Manage sites and assign multiple sites to the study. These can be sites within the Penn system, or outside collaborators. Multiple sites give patients and volunteers an option to select a research site closest to them
- Recruitment Campaign Tracker: Track the success of your recruitment campaign (i.e., flyer, TV ads, radio, etc.) real-time and longitudinally using a trackable phone number and email.
- Website Creation*: web-based, using a few clicks
- Volunteer Registry*: Find potential participants via the OCR-iConnect registry using your study condition terms. No separate IRB approval required to use Volunteer Registry.
- Adding In-clinic Patients: Ability to add in-clinical patients or referrals that have volunteered through the study listing.
*Use of these features requires IRB approval at time of study submission. Please work with OCR for template IRB wording for these features. OCR can also help with brainstorming on how to operationalize these features to enhance recruitment.
SOC 2 Certified
SOC 2 (System and Organization Controls 2) is a widely recognized auditing standard developed by the American Institute of CPAs (AICPA). It focuses on a service organization's non-financial reporting controls as they relate to security, availability, processing integrity, confidentiality, and privacy of customer data. SOC 2 certification demonstrates highest levels of security and data protection. Read the press release for more information on SOC 2.
Link to the iConnect platform for study teams and patients: https://clinicalresearch.pennmedicine.org/
Penn Medicine research staff access iConnect using PennKey. Training is required to activate access.
Questions or Concerns? Contact Office of Clinical Research:
- Phone: 215-662-4484
- Email psom-ocr@pobox.upenn.edu or Recruitment Specialist: thohing@upenn.edu or mdutt@upenn.edu
Advance (Consenting, ePRO thru Mobile Apps)
About
The Advance platform allows research teams to create consents in a mobile app creation platform, including consent review, and e-signatures.
Advance is a highly configurable Do-It-Yourself solution to collect research data from any source. This platform is powered through the Vendor, TrialX.
Consider these before choosing:
-
Platform is HIPAA compliant,
-
21 CFR Part 11 capable, but not certified as compliant.
-
It is designed for ePRO data collection from multiple sources, including wearables, images.
-
These is a cost for this application, it is subsidized for Penn Medicine research teams.
-
Platform allows API integration with others such as REDCap
-
Penn IS approved platform with Business Associates Agreement
Access & Training
OCR will meet with each team to understand needs from the application.
Resources
See more information on Advance here: https://trialx.com/xpand/
Support
Please reach out to the Office of Clinical Research (C/o Maha Dutt, Tom Hohing – OCR Operations) for demo, consult, training and access.
Outreach Using CureTalks@Penn
CureTalks@Penn is a research interview podcasts service offered through the Office of Clinical Research.
These interviews help research teams conduct wider outreach about their research trials or initiatives, or spread the word about hard to recruit, rare disease trials.
CureTalks are offerred to Penn research teams at no cost.
The interview is typically completed virtually with the study PI and 2 or 3 subject experts. The interview is then edited and placed on Penn Medicine's website, social media platforms and YouTube.
If the interview is created and presented to aid recruitment for a specific trial, the interview script requires IRB approval.
Click on the Resource link to see a list of curated interviews since 2018.
To inquire about producing a CureTalks interview contact the OCR:
- Phone: 215-662-4484
- Email psom-ocr@pobox.upenn.edu or Recruitment Specialist: thohing@upenn.edu or mdutt@upenn.edu