Oversight & Study Team Members
Penn Highlights in Clinical ResearchPI Oversight and Personnel Resources
For some studies, an individual might be both the PI and the (Regulatory) Sponsor. In this case, both sets of responsibilities mentioned apply.
Content coming soon.
Sponsor Oversight and Personnel Resources
For some studies, an individual might be both the PI and the (Regulatory) Sponsor. In this case, both sets of responsibilities mentioned apply.
Sponsor Team Roles
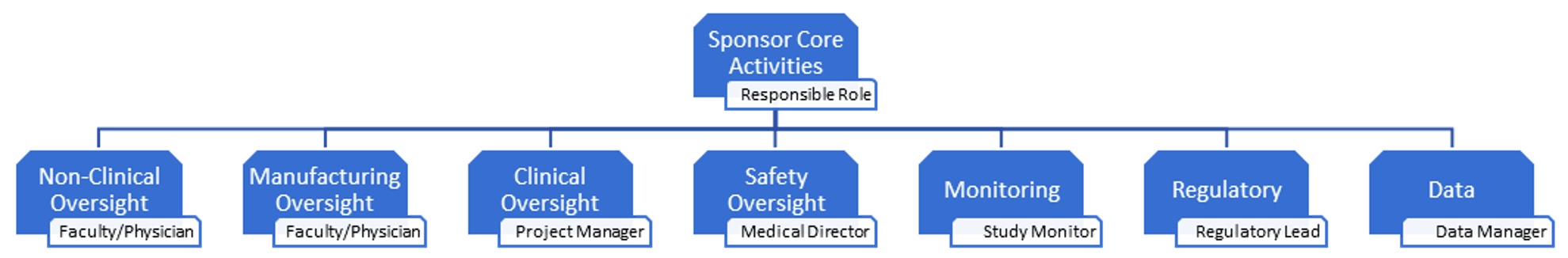
By far the most important part of being a Sponsor is compiling a qualified trial team that is complimented, as needed, by vendors to fulfill all activities that satisfy the Principles of ICH GCP. The sponsor team should consist of the appropriate number of qualified individuals with sufficient expertise in the area they will lead.
Below is a description of the core activities and the name assigned to the individual leading it:
The team and trial operations should be governed by written procedures such as standard operating procedures (SOPs). The PSOM has established Sponsor SOPs for Sponsor Responsibilities, Vendors, Investigational Product, Pharmacovigilance, Monitoring and Data Management. Your Trial Team list as well as documentation of trial team training and qualifications, including Financial Interests, should be documented in your TMF.
Sponsor team training requirements outlined by PSOM can be found in SOP 401 and SOP 006; however, the Sponsor should determine if additional training is required to fulfill assigned roles(e.g., SOP 004 for the Monitor, SOP 005 for the Medical Director, SOP 007 for the Data Manager). Sponsor teams may require additional SOPs and should develop trial support systems and tools specific to the team and trial. Some examples of these tools include documents that should be maintaining in your TMF such as an Operations Manual, Communication Plan, Roles and Responsibility Matrix and Operational Oversight/Study Decision Log
If you would like any support or assistance, please contact OCR Regulatory at PSOM-IND-IDE@pobox.upenn.edu.