Investigational Product Management-Study Start Up
The management of an Investigational Product (IP) is a complex and highly regulated activity. The IP may be a drug, biologic, medical device, or combination product; each of which may have unique regulatory and accountability requirements. If the investigator is also serving as the sponsor, additional regulations apply as they are ultimately responsible for the product.
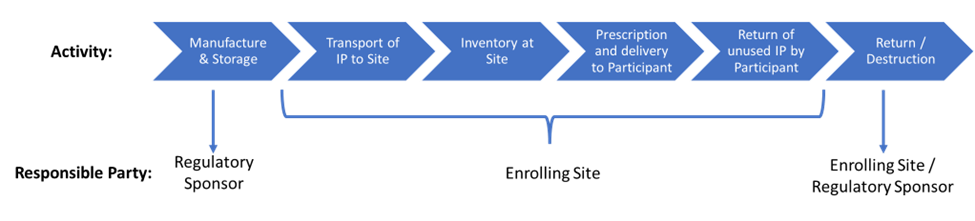
Being accountable for the IP includes, but is not limited to, documenting the conditions under which the test article was shipped, stored, administered, returned, and/or destroyed, along with any special labeling or quality control measures that may be required. The research team must also be trained on proper administration of the IP as well as any protocol related requirements for its safe and appropriate use such as randomization and un-blinding procedures.
It is the responsibility of the site Principal Investigator (PI) of a clinical trial to ensure the accurate and complete accountability including the proper storage and disposition of investigational products during the lifecycle of the research. This PowerPoint slide deck Penn Medicine One Research Site IP Managment Study Start Up educates site investigators and staff on the required elements of IP accountability management during the study start-up/initiation phase of a trial.
At Penn all investigational drugs should be maintained by the Investigational Drug Services. Please consult with them for more information about storage, handling, labeling, dispensing, randomization and unblinding at: Institute for Translational Medicine and Therapeutics - Investigational Drug Service (IDS).
Research (investigational) medications may refer to any novel therapies (vaccines, biologics, drugs, etc.) or commercially available medications ordered or administered as part of a research protocol.
- All research (investigational) medications for research participants being seen at Penn Medicine locations are to be electronically ordered via PennChart.
- All research (investigational) medications being administered to research participants at Penn Medicine locations are to be documented in PennChart.
- All research studies with research (investigational) medications ordered or administered as part of a research protocol must have a research study “RSH” record in PennChart.
Procedures for requesting research (investigational) medication build in PennChart:
Beacon Protocol Request:
Study team submits Protocol build request form* to PennChart Beacon team using the following link Beacon Protocol Build Request
Pharmacist submits a medication build request form to the Beacon Team using the following link Beacon or Research Medication Build Request.
*The applicable request form (Protocol Build Request or Investigational Medication Build Request) is included in the Athena ticket using the hyperlinks listed above.
Non-Beacon Medication Request:
Pharmacist or Study team submits a medication build request form* to the PennChart Research Team using the following link Beacon or Research Medication Build Request
*The applicable request form (Investigational Medication Build Request) is included in the Athena ticket using the hyperlink above.
Selecting & Qualifying your Investigational Product Vendor:
A clinical trial may include investigational and study products. When selecting and qualifying a manufacturing vendor (or supplier) you should consider the regulatory requirements that apply to the planned trial. The vendor may be a Contract Manufacturing Organization, a Penn Manufacturer, an Industry Partner, or a marketed product that can be purchased through the Pharmacy (Investigational Drug Services- IDS).
For clinical trials that have IPs, please refer to PSOM SOP 003 Investigational Product Management for additional details on oversight of the manufacturing. The Sponsor must retain all Essential Documents demonstrating oversight and control of the investigational product in the Trial Master File. If you require assistance with qualification of a manufacturer or have questions, please contact OCR Regulatory at PSOM-IND-IDE@pobox.upenn.edu
If you intend to utilize the services of Penn IDS for the management of your investigational product, you can contact them or utilize the forms on their website for specific requests (cost estimates, medication requests, and destruction of clinical trial information.
Manufacturing Quality:
The following decision tree will help with deciding the standards which should be applied for Drugs based on the phase of your clinical trial:
Sponsor Manufacturer Quality Standard Deision Tree for Drugs and Biologics

Drugs and Biologics
- Current Good Manufacturing Practice for Phase 1 Investigational Drugs
- 21 C.F.R. § 210- Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs; General
- 21 C.F.R. § 211- Current Good Manufacturing Practice for Finished Pharmaceuticals
Radiopharmaceuticals
Devices
-
1204
- Quality System (QS) Regulation/Medical Device Current Good Manufacturing Practices (CGMP)
- 21 C.F.R. § 820- Quality System Regulation
International Organization for Standardization