Study Close Out
PI Study Close Out
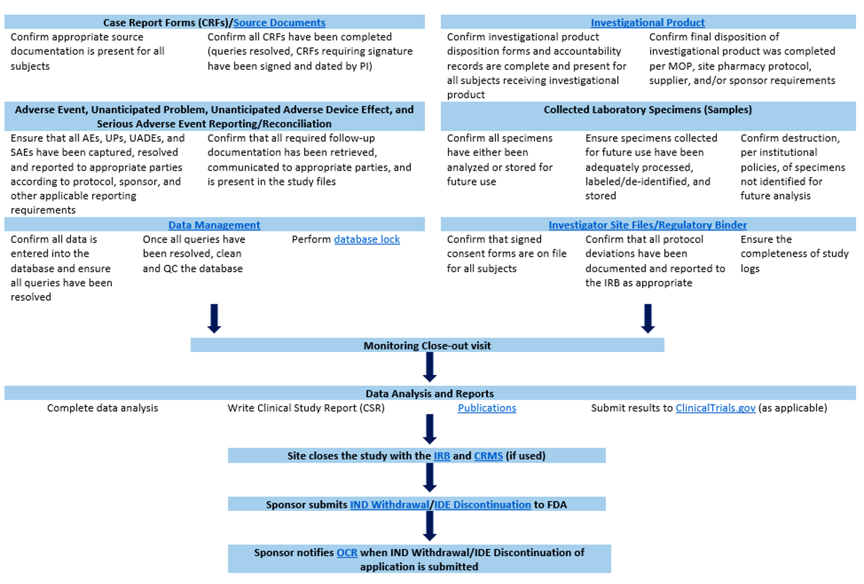
After enrollment is closed, all follow-up data has been collected, utilization of subject PHI is no longer required, and the final data analysis is complete, the study is considered to be in the Closeout Phase. During this phase, data is prepared for final analysis, regulatory and reviewing bodies are notified of study closure, investigational product inventory is reconciled, and final reports are submitted. Also, contact the following areas to ensure system are properly closed.
- IRB
- Clinical Research Finance (PennChart record and residual balance transfers)
For some studies, an individual might be both the PI and the (Regulatory) Sponsor. In this case, both sets of responsibilities mentioned apply.
Sponsor Study Close Out
Ultimately, the Sponsor assesses all of the areas below and decides when a study is complete. Once the Sponsor considers the study to be complete, the Sponsor notifies all involved parties, as detailed below.
For some studies, an individual might be both the PI and the (Regulatory) Sponsor. In this case, both sets of responsibilities mentioned apply.
Click the image to download