
Central Nervous System (CNS) Imaging Laboratory

Director of the CNS Imaging
Associate Professor of Ophthalmology and Radiology
ashtari@upenn.edu
Dr. Ashtari is the director of the Central Nervous System (CNS) Imaging Laboratory at CAROT. The CNS imaging laboratory is involved in a multi-disciplinary neuroimaging research to explore the mechanisms underlying neuronal bases of visual processing in patients with low vision and the subsequent remodeling of the visual pathways upon regaining vision through retinal gene therapy.
The CNS laboratory incorporates advanced and unique methods of magnetic resonance imaging (MRI) techniques including functional MRI (fMRI), diffusion tensor MRI (dMRI), and resting state fMRI (rsfMRI). Employing dMRI in conjunction with fMRI technique, our research has successfully demonstrated that functional and structural aspect of the human visual pathways are substantially remodeled following vision restoration.
At CAROT, the neuroimaging project builds upon expertise across multiple fields of science and medicine with a unique access to group of patients regaining vision through gene therapy to gain a deeper understanding of the partnership between the brain and retina and its potential to uncover the underlying process of human brain plasticity. This type of knowledge on brain plasticity of visual systems currently comes largely from animal models with visual impairment followed by vision restoration. Based on our preliminary data, our hope is to develop innovative rehabilitation program that are centered on an alternate brain pathway (used by low vision patients) to enhance visual function in patients who are not currently candidate for retinal gene therapy.
The CNS lab has participated in all three phases of the Leber’s congenital Amaurosis (LCA) clinical trials that have taken place at The Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania (Upenn). We were the first to describe the restoration of visual pathways in humans after retinal gene therapy 1 .
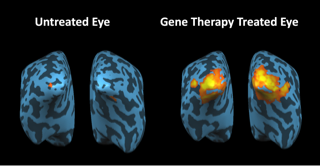
This study demonstrates that despite severe and long-term visual impairment, treated LCA2 patients have intact and responsive visual pathways (see Figure 1). In addition, our study also showed that gene therapy resulted in not only sustained and improved visual ability, but also enhanced contrast sensitivity.
Unanticipated findings are also recognized and evaluated further in a scientific manner. For example, we have noted that some of the subjects with LCA show highly unusual fleeting activations in the brain that do not correspond to administration of visual stimuli (see Figure 2).
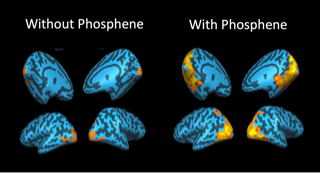
By carefully investigating the reports of subjects about these “phosphenes,” and correlating the phosphene experiences with her imaging/fMRI results, we showed that phosphene experiences are quite common in patients with LCA and that they can distort the interpretation of various clinical tests 2. This is important information for retinal degeneration specialists as these doctors usually do not query about phosphine experiences. Unanticipated findings are also recognized and evaluated further in a scientific manner. For example, we have noted that some of the subjects with LCA show highly unusual fleeting activations in the brain that do not correspond to administration of visual stimuli (see Figure 2). By carefully investigating the reports of subjects about these “phosphenes,” and correlating the phosphene experiences with her imaging/fMRI results, we showed that phosphene experiences are quite common in patients with LCA and that they can distort the interpretation of various clinical tests 3. This is important information for retinal degeneration specialists as these doctors usually do not query about phosphine experiences.
Using non-invasive multimodal neuroimaging methods, we also demonstrated that reversing blindness with gene therapy promoted long-term structural plasticity in the visual pathways emanating from the treated retina of LCA patients. The data revealed improvements and normalization along the visual fibers corresponding to the site of retinal injection of the gene therapy vector carrying the therapeutic gene in the treated eye compared to the visual pathway for the untreated eye of LCA patients 4. After gene therapy, the primary visual pathways (for example, geniculostriate (GS) fibers) in the treated retina were similar to those of sighted control subjects, whereas the primary visual pathways of the untreated retina continued to deteriorate.
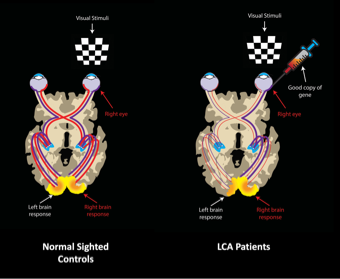
This is pictorially demonstrated in Figure 3. The GS fibers provide the primary visual information from the retina to the visual cortex via the lateral geniculate nucleus (LGN). However, the human visual cortex is also connected to many other brain regions (see Figure 4) to provide higher order visual information. Diffusion tensor tractography analyses of all of these fibers showed no difference between the LCA2 patients and sighted controls except for the GS fibers (Ashtari et al, 2015).
Currently, CNS Lab is carrying out studies on patients enrolled in Phase III of the LCA gene therapy trial. These individuals are evaluated at baseline and at prescribed timepoints, 1, 3, 6 months and annually for five years, following intervention and some of the individuals are randomized to a control group prior to intervention. The data being collected are vast and are being used to evaluate numerous hypotheses.

To further study the dynamic transformations of the human primary visual cortex microstructure, prompted by the retinal gene therapy, a more advanced method of dMRI known as the Neurite Orientation Dispersion & Density Imaging (NODDI) has been employed by the CNS lab. Using the cutting-edge methods of image analyses developed by the Penn Image Computing & Science Lab (PICSL) and the NODDI imaging we have captured the timing when the microstructural changes of the visual cortex after retinal gene therapy is peaked Our data is suggestive of a critical time window for maximal changes in the primary visual cortex dendritic trees, which may in turn may play a critical role in designing post retinal surgery rehabilitation strategies 5. We strongly believe an efficient visual rehabilitation program administered at a critical time after vision restoration would play an immense role in increasing vision in patients who are receiving gene therapy (or other vision restoration techniques) to further augment their visual functions.
The LCA Phase III patients also undergo the powerful method of resting state fMRI (rsfMRI) and several hypotheses regarding the analysis of this data is currently underway.
Choroideremia Clinical Trial
The CNS lab is also engaged in acquisition and analyses of data from a group of patients with Choroideremia (CHM) and demographically matched sighted controls. The CHM patients are recruited from a Phase-I clinical trial retinal gene therapy study currently ongoing at CHOP/Penn. A subset of patients from this clinical trial have been followed up to three years in a separate neuroimaging study. In addition to the imaging protocol described here for the LCA2 study, CHM patients underwent additional fMRI protocol to map their visual field called population receptive field (pRF). The results from this study was recently published in the journal of Investigative Ophthalmology & Visual Science (Silson et al, 2018).
References
1. Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS, Bennett J. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 121(6):2160-8, Jun 2011.
2. Ashtari M, Cyckowski L,Yazdi AViands, AMarshall K,Bókkon I, Maguire A, Bennett J: fMRI of retina-originated phosphenes experienced by patients with Leber congenital amaurosis. PLoS One 9(1), Jan 2014.
3. Ashtari M, Zhang H, Cook PA, Cyckowski LL, Shindler KS, Marshall KA, Aravand P, Vossough A, Gee JC, Maguire AM, Baker CI, Bennett J. Plasticity of the human visual system after retinal gene therapy in patients with Leber's congenital amaurosis. Sci Transl Med 7(296), Jul 2015.
4. Lipin MY, Godbole T, Cook P, Willett A, Yu Y, Ying GS, Maguire A, Bennett J, Zhang G, Ashtari M. Effects of sight restoration on the dendritic complexity of the primary visual cortex. Society for Neuroscience Meeting, San Diego, CA, 2018, Program #143.12
5. E. H. Silson, Tomas S. Aleman, Aimee Willett, Leona W. Serrano, Denise J. Pearson, Andreas M. Rauschecker, Albert M. Maguire, Chris I. Baker, Jean Bennett and, Manzar Ashtari. Comparing Clinical Perimetry and Population Receptive Field Measures in Patients with Choroideremia. IOVS July 2018 Vol. 59 No. 8.
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser. | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: PMACS Web Team.
