Penn Medicine REDCap
REDCap was created by Vanderbilt University and is available at Penn Medicine via a license agreement. Penn Medicine’s REDCap is a HIPAA-compliant web platform for building and managing online databases and surveys. REDCap's streamlined process for rapidly creating and designing projects offers a vast array of tools to fit many data collection strategies, such as single-/multi-site studies, longitudinal studies, cross-sectional studies, collecting data via surveys, and/or a combination. REDCap provides automated export procedures for seamless data downloads to Excel and common statistical packages (e.g., SPSS, SAS, Stata, R).
IMPORTANT NOTES
- Only Penn Medicine’s REDCap instance is HIPAA-compliant
- Penn Medicine’s REDCap is NOT a 21 CFR Part 11-compliant system and should not be used for studies reporting to the FDA (i.e., IND, IDE, abbreviated IDE, IND-exempt, and IDE-exempt).
Penn Medicine REDCap Account Creation
Users Internal to Penn/Penn Medicine
To have a Penn Medicine REDCap account created, users associated with the University of Pennsylvania and/or Penn Medicine (UPHS/PSOM) must have: 1) an active PennKey; and 2) an active PMACS account.
If you are associated with UPenn and/or Penn Medicine, and you do not have an active PMACS account (or don’t know what one is), please refer to their website.
Users with the above requirements must complete the below steps
- Complete the required eLearning via the user’s Learning Management System (LMS)
- eLearning title: “REDCap: Best Practices for Maintaining HIPAA Compliance and Protecting Personal Health Information (PHI)”
- eLearning’s links for each LMS:
- Request account creation via PSOM REDCap Support’s MACHFORM
Users External to Penn/Penn Medicine (non-Penn staff)
Non-Penn employees/external users will need a Guest PennKey. External users affiliated with CHOP need a “CHOP” PennKey.
A PennKey is a unique username required to authenticate your identity for access to many of Penn’s online resources and systems, including PMACS accounts, Penn Medicine REDCap, and Learning Management System access. To obtain a Guest or CHOP PennKey, external users must be “sponsored” by a Penn faculty or staff member.
External users should follow these steps
- The external user’s Penn “sponsor submits the request for a Guest PennKey
- For CHOP PennKey Access, click here
- Complete the required eLearning via the user’s Learning Management System (LMS)
- eLearning title: “REDCap: Best Practices for Maintaining HIPAA Compliance and Protecting Personal Health Information (PHI)”
- eLearning’s links for each LMS:
- Workday Learning
- If you do not have access to Workday Learning, submit a Solutions Center ticket indicating “Learning Support” as the ticket category and the Workday Learning training you need.
- Knowledge Link
- Workday Learning
- Request account creation via PSOM REDCap Support’s MACHFORM
Access for existing Penn Medicine REDCap Accounts
Log in to the Penn Medicine REDCap website using your PMACS account username and password.
If you are unable to log into your account, please contact PSOM REDCap Support (MACHFORM). Provide as much information as possible when completing the form.
Policy Documents
SOP 405: REDCap Training and Access (Standard Operating Procedure)
Penn Medicine REDCap Training
Once your Penn Medicine REDCap account is created, watching all REDCap training videos is STRONGLY RECOMMENDED before building your first project.
OCR hosts three, monthly training courses for using Penn Medicine REDCap.
REDCap 101
REDCap 101 instructs users on basic REDCap functionality and building REDCap projects. After completing this 90-minute, instructor-led course, users will obtain a solid foundation on building REDCap projects that result in cleaner data sets. Topics include reviewing basic functions (i.e., field types, validation, etc.), using branching logic and Action Tags, designing REDCap surveys, and reviewing exported project data.
For more information, see the blended course offering in Workday Learning.
REDCap 201
Building on the basics covered in REDCap 101, this 3-hour, instructor-led course provides instruction on more complex REDCap functionality. Topics include a more in-depth review of survey functionality, survey invites, creating longitudinal projects, and how to use REDCap’s Data Resolution Workflow to create queries on project data. To register, users must complete the REDCap 101 course and have an active Penn Medicine REDCap account.
For more information, see the blended course offering in Workday Learning.
REDCap was created by Vanderbilt University and is available at Penn Medicine via a license agreement between these two institutions. Additionally, REDCap is available at many institutions around the world using these agreements. This makes finding resources easy by using search engines. However, each organization can customize their REDCap system. It’s important to understand this because functionality described by another institution may not be available in Penn Medicine’s REDCap or may not work exactly the same.
The resources provided here are specific to Penn Medicine’s REDCap.
Penn Medicine REDCap Support
For login issues and/or questions regarding REDCap functionality/troubleshooting, email the REDCap Support Team. Alternatively, you can also use this REDCap Support Help Form (no login required).
For issues with your PMACS credentials, or to reset your PMACS password, please go to PMACS Password Reset.
FAQ
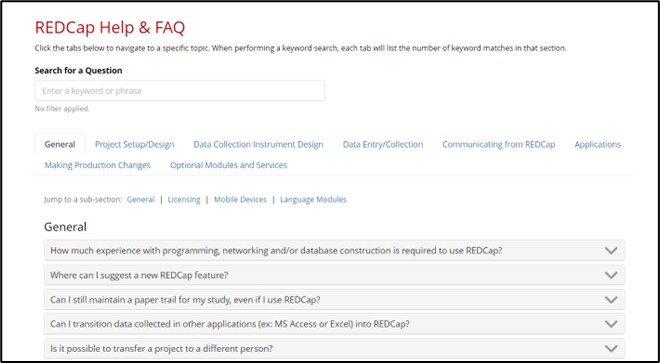
REDCap provides an in-site FAQ module.
After logging in, look for the “Help & FAQ” link in the menu at the top of the screen.

All FAQs will appear. You can scroll through all FAQs or click the tab that groups specific FAQs together. You can also use the search function for specifics.
Penn Medicine REDCap is an electronic data capture tool that can be used for capturing informed consent documentation electronically for studies that do not require Part 11 compliant signatures.
Consider before choosing Penn Medicine REDCap for e-Consenting:
-
Application cannot be used for studies reporting to the FDA; this includes the below study types:
- INDs
- IDEs
- Abbreviated IDEs
- IND-exempt
- IDE-exempt
- Study team members must have active PMACS and Penn Medicine REDCap accounts
- E-Consent projects must be created in Penn Medicine REDCap only
Benefits:
-
Application is HIPAA compliant for e-signatures.
- Free - no cost associated with use of Penn Medicine REDCap.
- Easy self-service workflows.
- Upload UPenn IRB - approved consent
- REDCap surveys allow:
- Participants review and electronically sign informed consents
- Study team representatives electronically sign their attestation of informed consent
- All e-signed informed consent documentation is stored in REDCap in PDF format.
- Providing participant access to their e-signed informed consent documents using REDCap’s “Send-It” function
Guidance & Project Template
OCR Operations has developed the below guidance document as well as a REDCap e-Consent project template. The guidance document contains all information needed for creating and managing REDCap e-Consent projects, such as, how to customize the project template to create an e-Consent project for your research study, add new informed consent versions, and creating tracking reports..
The REDCap project template can be found in the project templates table on the “+Create a new REDCap Project” page. The guidance document contains instructions to access the template. This template provides the needed forms, surveys, and alerts to capture eConsents for a single-site research study enrolling participants capable of providing informed consent both mentally and physically.
Penn Medicine REDCap (REDCap) is a HIPAA-compliant EDC system with functionality to electronically document their Delegation of Authority Logs (eDoA) for research protocols. This allows research teams to electronically document:
- Study role & responsibilities for all study personnel
- Study personnel acknowledgment of their study responsibilities
- Principal Investigator (PI) certification of study personnel’s responsibilities
- PI certification that study personnel is no longer associated with the study
By using REDCap surveys, active REDCap accounts are not required by study’s personnel or the PI to acknowledge and certify study responsibilities. NOTE: An active REDCap account is required for the study personnel responsible for the creation and maintenance of the eDoA REDCap project.
Considerations before using:
- Cannot be used for studies reporting to the FDA, this includes the below study types:
- INDs/IND-exempt
- IDEs/IDE-exempt/Abbreviated IDEs
- Only use the Penn Medicine REDCap to create eDoA Tracking projects
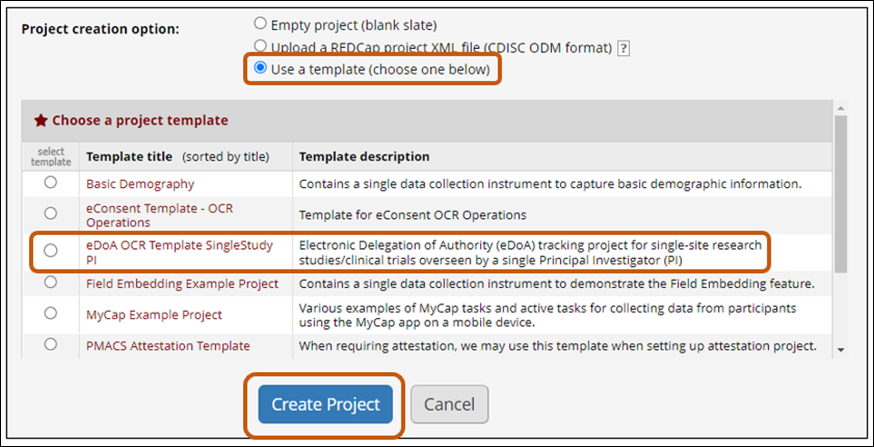
To assist the Penn Medicine research community and create a standard workflow, please use the OCR-created REDCap Template project (“eDoA_OCR_Template_SingleStudy_PI”) and the below guidance document.
This Template project contains all fields and functions needed for eDoA Tracking. Study teams must make a few modifications to fit the template to the research study; these are described in the guidance document.
To use the Template project:
- Login to Penn Medicine REDCap (https://redcap.med.upenn.edu/).
- Click the “+ New Project” link in the top navigation menu.

- Provide project’s title & purpose.
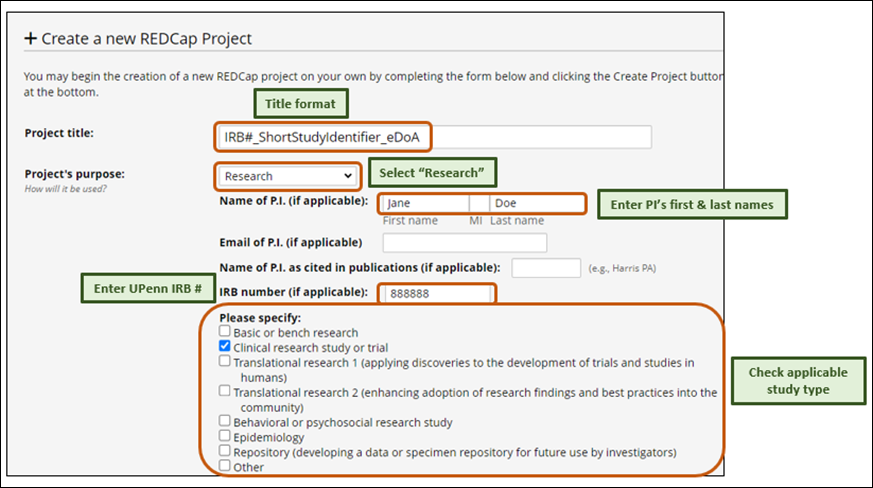
- Project creation option: select “Use a template (choose one below)”.
- Select “eDoA OCR Template SingleStudy PI”.
- Click “Create Project” button.
Electronic Delegation of Authority (eDoA) Tracking in Penn Medicine REDCap
The guidance explains the template project’s standard workflow and provides instructions for:
- Revising the template project to fit your research study.
- Tracking project’s data.
- Making post-Go-Live project revisions.
Currently, the template and guidance are intended for eDoA tracking projects used for single-site research projects conducted by a single Principal Investigator. We will provide templates and guidance for other study types (e.g., multi-site studies) in the future.
MyCap is a participant-facing mobile application used for data collection and the automated administration of Active Tasks (i.e., activities performed by participants using mobile device sensors under semi-controlled conditions). All data collected in the MyCap App are automatically sent back to the Penn Medicine REDCap server as soon as internet connection is available (i.e., it can also be used for offline participant data collection). This no-code solution supporting research teams conducting longitudinally designed projects or projects with frequent participant contact. MyCap also facilitates participant engagement and retention by providing quick access to project staff and two-way communications (e.g., messaging and announcements) within the App.
The use of MyCap to capture participant data should be explained in IRB applications and documented in research protocols for all Penn Medicine research projects. Template language can be found in REDCap’s “MyCap Help Document” (Penn Medicine REDCap login required for access).
Before building a MyCap App project, we recommend thoroughly reading the MyCap Help document and reviewing the resources on the Project MyCap website.
The below guidance supplements the information within the MyCap Help document. This guidance breaks down the process of building a MyCap project into logical steps and provides numerous screenshots. We recommend following them in the order provided.
General
The below tip sheets are related to general REDCap application use.
- Quick Start Guide: REDCap_QuickStart.pdf
- Avoiding Common Mistakes: REDCap_TipSheet_CommonMistakes.pdf
Data Management
Tip Sheets for managing project data.
- Querying Project Data – Data Resolution Workflow: REDCap_TipSheet_QueryingInREDCap_DRW
Data Reports/Exports
Tip Sheets on data reports and exports.
-
Protecting PHI in Data Exports: REDCap_TipSheet_PHI_DataExports
User Management
Tip Sheets on managing REDCap project users.
- User Rights Best Practices: REDCap_TipSheet_UserRights.pdf
- User Rights/Roles & PHI Access: REDCap_TipSheet_UserRights_PHI.pdf
Penn Medicine REDCap Support
For account-related issues, the preferred contact method is PSOM REDCap Support’s MACHFORM
Ament Group, within the Clinical Research Collaboration Unit, provides expert, fee-for-service support to investigators and research teams at the University of Pennsylvania. From basic builds to advanced automations, we deliver high-quality REDCap projects so you can focus on your science.
REDCap Development Group
Are you a researcher at Penn in need of a REDCap project but short on time or technical support?
The REDCap Development Group, within the Clinical Research Collaboration Unit, provides expert, fee-for-service support to investigators and research teams at the University of Pennsylvania. From basic builds to advanced automations, we deliver high-quality REDCap projects so you can focus on your science.
Why Choose the REDCap Development Group?
|
Get your project built right the first time
|
Services Provided
|
Service |
Description |
|---|---|
|
Basic Development |
Project setup, form/survey creation, branching logic, smart text |
|
Advanced Development |
Longitudinal design, automation, scheduling logic, data validation |
|
Build Review |
Refinement of existing or partially built projects |
|
External Modules |
Installation, configuration, troubleshooting |
|
Mobile & Messaging |
Mobile app setup, MyCap, text messaging |
|
Consent & Compliance |
eConsent development |
|
Reporting & Automation |
Dashboards, alerts, calculated fields, custom reports |
|
Randomization |
Stratified/block randomization setup |
|
Survey Development |
Multi-language, dynamic logic, automated survey invitations (ASI) |
|
Researcher Support |
Tailored training and consultations |
|
EPIC Integration |
Clinical Data Pull (CDP) from EPIC |
|
Account Management |
User accounts, roles and privileges, data access groups |
Standard Rate: $110/hour
Submits a request via https://redcap.link/redcapdev
Learn More: Visit https://www.cceb.med.upenn.edu/redcapdev
Contact: redcapdev@pennmedicine.upenn.edu

