Frontotemporal dementia
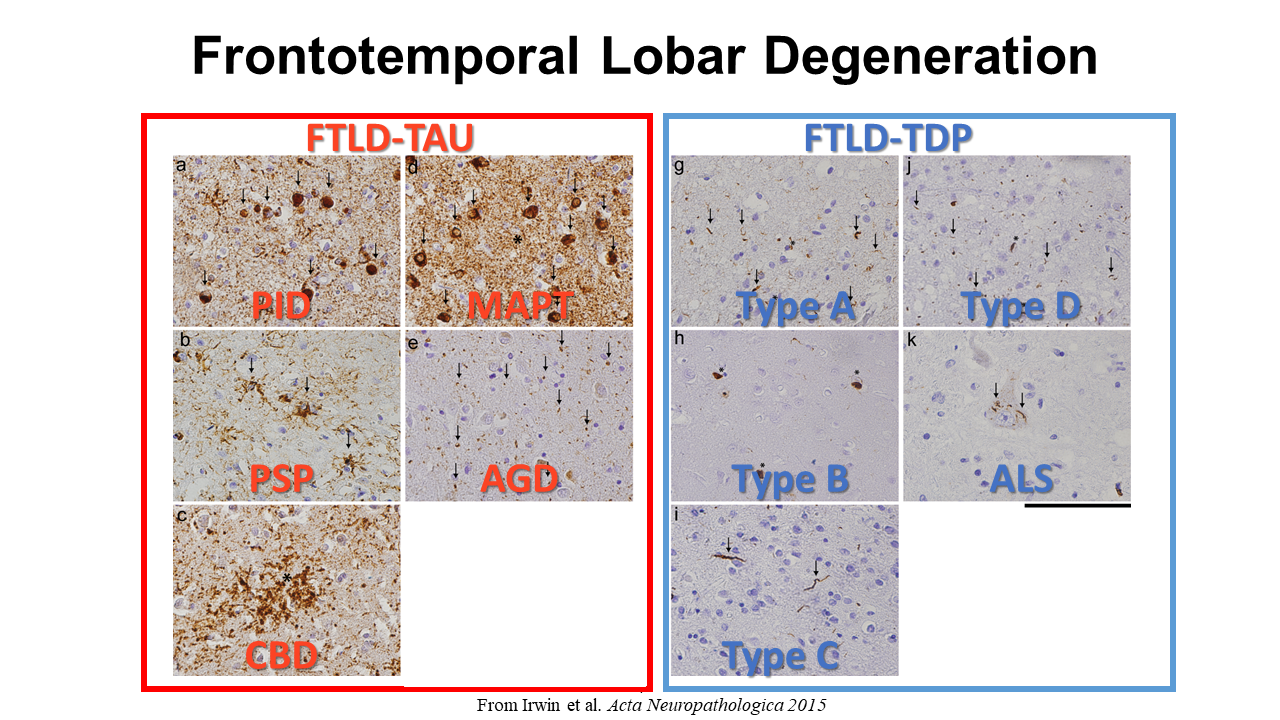
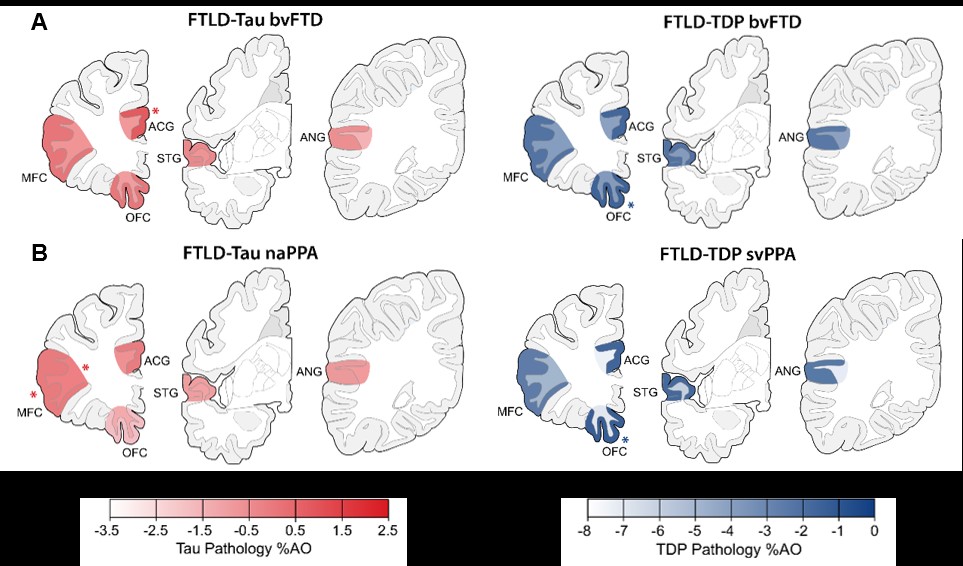
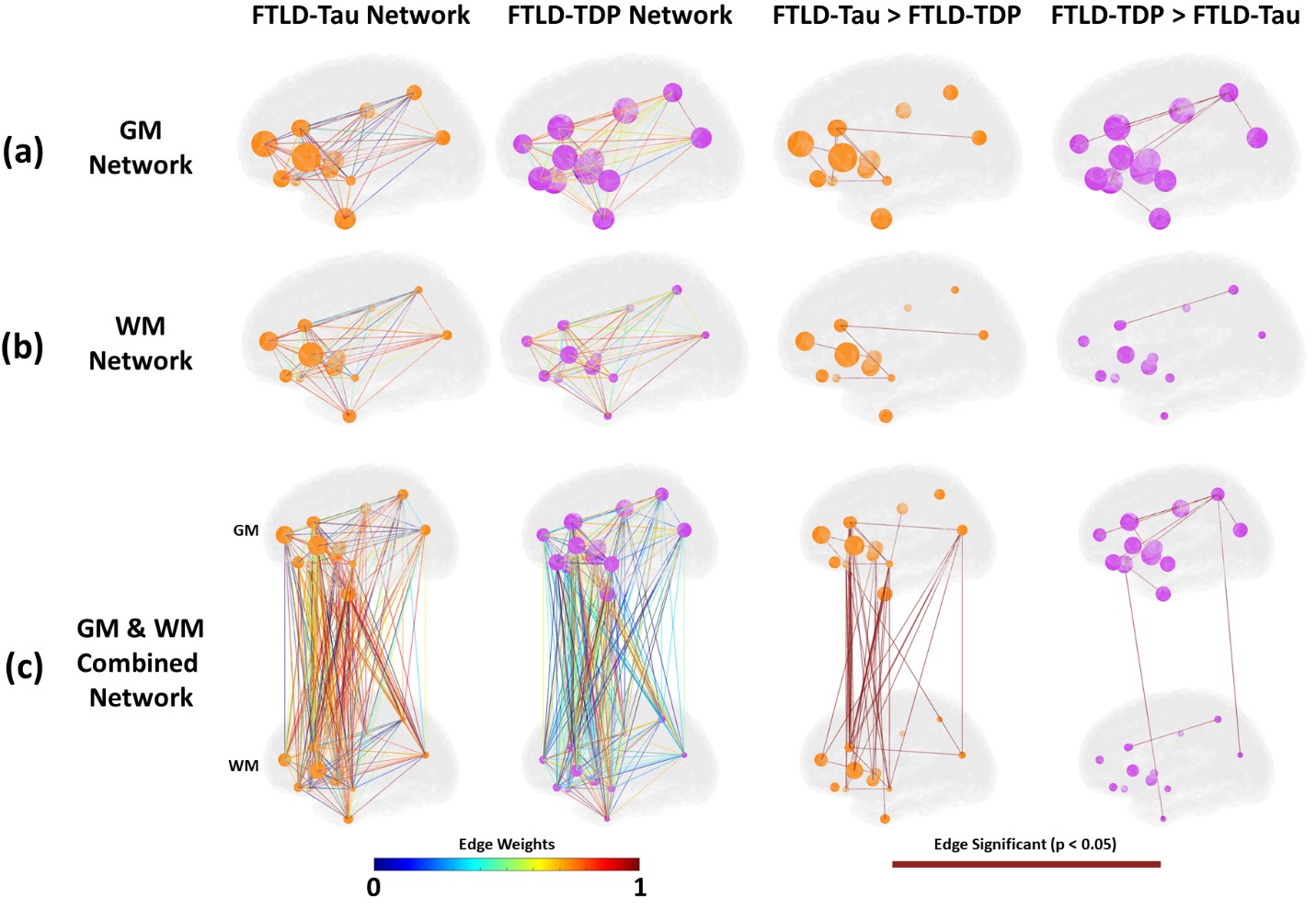
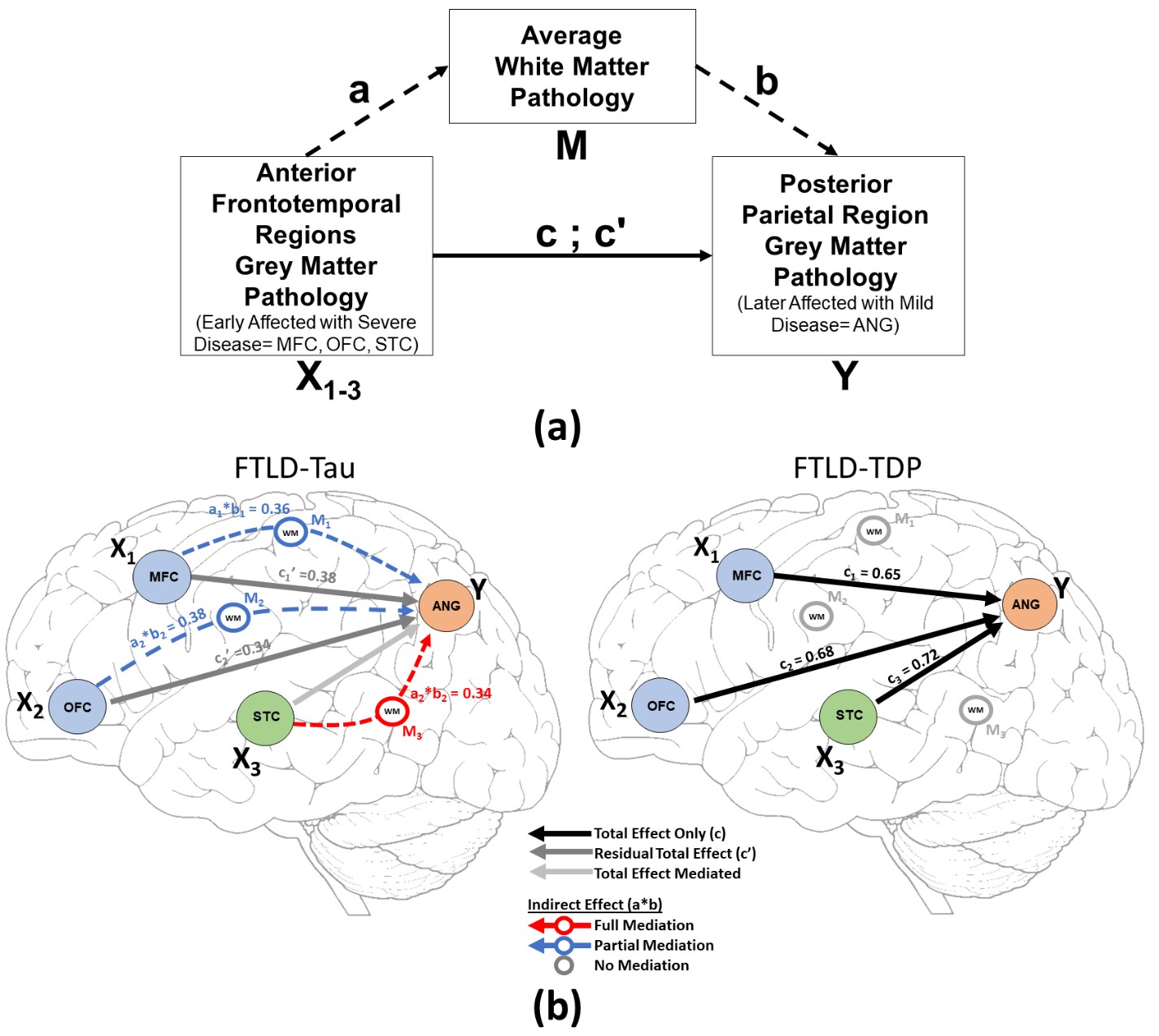
We hypothesize that each protienopathy associated with clinical FTD syndromes (i.e. FTLD-Tau, FTLD-TDP and AD – Figure 2) have distinct neuropathological patterns of neurodegeneration, both at a microscopic level and macroscopic regional distribution in the brain. We propose that these features can be used to develop tissue-specific imaging and biofluid biomarkers that can eventually be used in living patients to diagnose underlying neuropathologic diagnosis which is urgently needed for clinical trials. Indeed, using our digital histopathological measures we find divergent postmortem patterns of tau and TDP-43 pathology in grey and white matter across hemispheres in patients with bvFTD and PPA (Figure 3). We can use these digital metrics of microscopic disease burden to model disease propagation in the human brain using complementary approaches of network science (Figure 4) and mediation analyses (Figure 5). These data suggest a unique contribution of white matter disease in the spread of FTLD-Tau compared to FTLD-TDP. These human brain models of progressive disease will inform novel imaging biomarkers to detect and track FTLD proteinopathy in living patients.
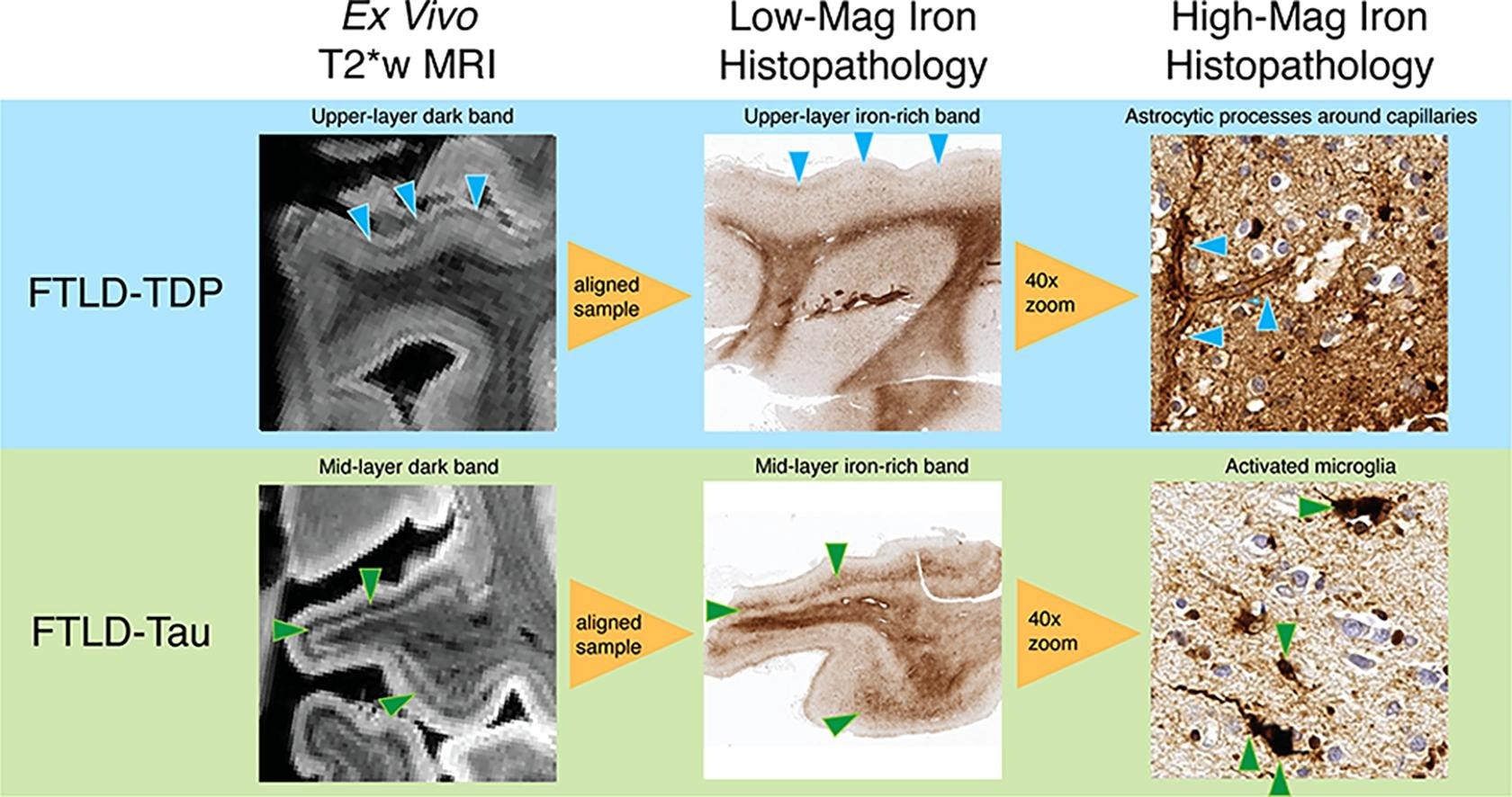
We also perform ultra-high resolution ex vivo 7T MRI imaging of the intact hemispheres. Using this approach we recently discovered distinct patterns of iron-rich gliosis in FTLD-Tau compared to FTLD-TDP (Figure 6) We can eventually adapt these sequences to perform a “virtual biopsy” using 7T MRI in living patients.
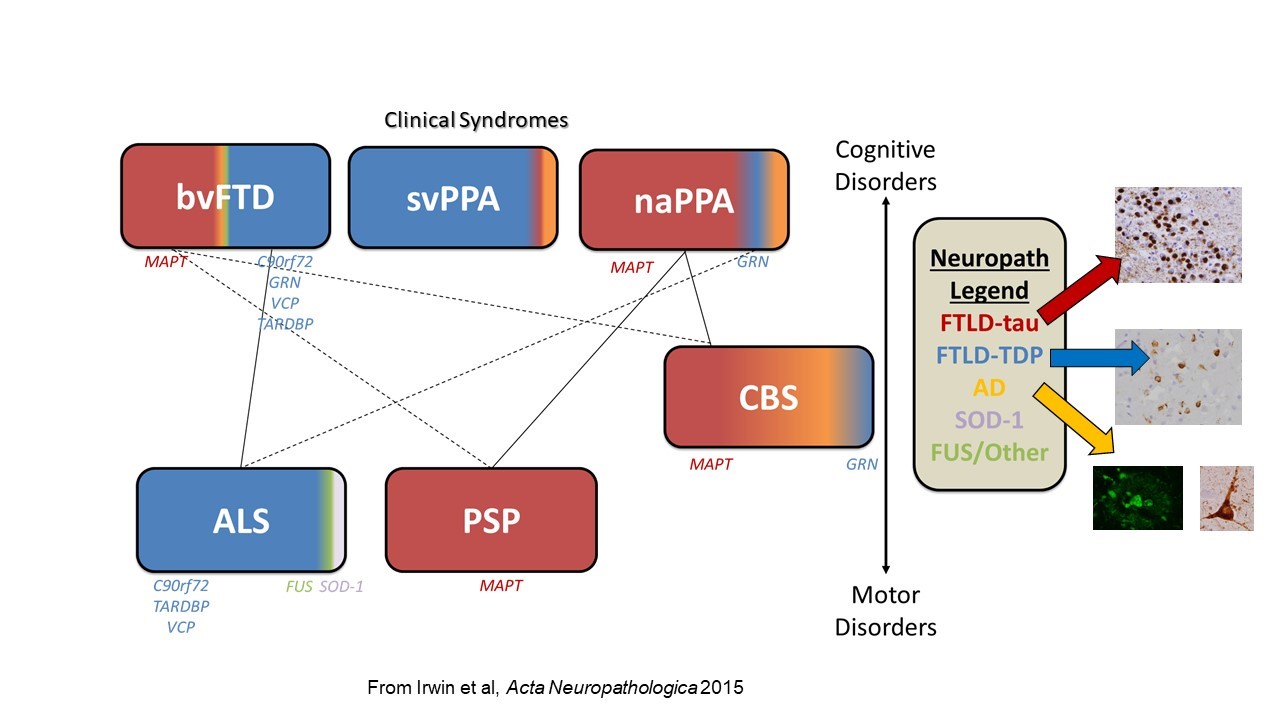
We hypothesize that each protienopathy associated with clinical FTD syndromes (i.e. FTLD-Tau, FTLD-TDP and AD – Figure 2) have distinct neuropathological patterns of neurodegeneration, both at a microscopic level and macroscopic regional distribution in the brain. We propose that these features can be used to develop tissue-specific imaging and biofluid biomarkers that can eventually be used in living patients to diagnose underlying neuropathologic diagnosis which is urgently needed for clinical trials. Indeed, we find divergent postmortem patterns of tau and TDP-43 pathology in grey and white matter across hemispheres in patients with bvFTD (Figure 3) and PPA (Figure 4), and our microscopic digital measures of pathology relate to antemortem structural imaging, suggesting an integrative approach to relate antemortem imaging to postmortem pathology will reveal novel insights into the patterns of spread of these distinct proteinopathies to cause clinical FTD. Please see our publications below which highlight our recent findings to support this overarching hypothesis.
-
Divergent Histopathological Networks of Frontotemporal Degeneration Proteinopathy Subytpes.
The Journal of neuroscience : the official journal of the Society for Neuroscience. 2022 May 4;42(18):3868-3877 Epub 2022 Mar 22; Authors: Chen M, Ohm DT, Phillips JS, McMillan CT, Capp N, Peterson C, Xie E, Wolk DA, Trojanowski JQ, Lee EB, Gee J, Grossman M, Irwin DJ -
Signature laminar distributions of pathology in frontotemporal lobar degeneration.
Acta neuropathologica. 2022 Mar;143(3):363-382 Epub 2022 Jan 8; Authors: Ohm DT, Cousins KAQ, Xie SX, Peterson C, McMillan CT, Massimo L, Raskovsky K, Wolk DA, Van Deerlin VM, Elman L, Spindler M, Deik A, Trojanowski JQ, Lee EB, Grossman M, Irwin DJ -
Degeneration of the locus coeruleus is a common feature of tauopathies and distinct from TDP-43 proteinopathies in the frontotemporal lobar degeneration spectrum.
Acta neuropathologica. 2020 Nov;140(5):675-693 Epub 2020 Aug 17; Authors: Ohm DT, Peterson C, Lobrovich R, Cousins KAQ, Gibbons GS, McMillan CT, Wolk DA, Van Deerlin V, Elman L, Spindler M, Deik A, Siderowf A, Trojanowski JQ, Lee EB, Grossman M, Irwin DJ - Ex vivo MRI and histopathology detect novel iron-rich cortical inflammation in frontotemporal lobar degeneration with tau versus TDP-43 pathology
Neuroimage Clin. 2022;33:102913. doi: 10.1016/j.nicl.2021.102913. Epub 2021 Dec 14; Authors: M Dylan Tisdall 1 , Daniel T Ohm, Rebecca Lobrovich, Sandhitsu R Das, Gabor Mizsei, Karthik Prabhakaran, Ranjit Ittyerah, Sydney Lim, Corey T McMillan, David A Wolk, James Gee , John Q Trojanowski, Edward B Lee, John A Detre, Paul Yushkevich, Murray Grossman, David J Irwin
- Giannini Lucia A A, Xie Sharon X, McMillan Corey T, Liang Mendy, Williams Andrew, Jester Charles, Rascovsky Katya, Wolk David A, Ash Sharon, Lee Edward B, Trojanowski John Q, Grossman Murray, Irwin David J: Divergent patterns of TDP-43 and tau pathologies in primary progressive aphasia. Annals of neurology 85(5): 630-643, May 2019. doi:10.1002/ana.25465.
- Irwin David J, McMillan Corey T, Xie Sharon X, Rascovsky Katya, Van Deerlin Vivianna M, Coslett H Branch, Hamilton Roy, Aguirre Geoffrey, Lee Edward B, Lee Virginia M Y, Trojanowski John Q, Grossman Murray: Asymmetry of post-mortem neuropathology in behavioural-variant frontotemporal dementia. Brain: a journal of neurology 141(1): 288-301, Jan 2018. doi:10.1002/ana.24996.
- Giannini Lucia A A, Irwin David J, McMillan Corey T, Ash Sharon, Rascovsky Katya, Wolk David A, Van Deerlin Vivianna M, Lee Edward B, Trojanowski John Q, Grossman Murray: Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology 88(24): 2276-2284, Jun 2017. doi:10.1212/wnl.0000000000004034.
- Irwin David J, Lleó Alberto, Xie Sharon X, McMillan Corey T, Wolk David A, Lee Edward B, Van Deerlin Viviana M, Shaw Leslie M, Trojanowski John Q, Grossman Murray: Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Annals of neurology 82(2): 247-258, Aug 2017. doi:10.1002/ana.24996
- Irwin David J, Cairns Nigel J, Grossman M, McMillan Corey T, Lee Edward B, Van Deerlin Vivianna M, Lee Virginia M-Y, Trojanowski John Q: Frontotemporal Lobar Degeneration: Defining Phenotypic Diversity through Personalized Medicine. Acta Neuropathologica, vol. 129, no. 4, 2014, pp. 469–491., doi:10.1007/s00401-014-1380-1
- Irwin David J, McMillan Corey T, Suh EunRan, Powers John, Rascovsky Katya, Wood Elisabeth M, Toledo Jon B, Arnold Steven E, Lee Virginia M-Y, Van Deerlin Vivianna M, Trojanowski John Q, Grossman Murray: Myelin oligodendrocyte basic protein and prognosis in behavioral-variant frontotemporal dementia. Neurology 83(6): 502-9, Aug 2014. doi: 10.1212/WNL.0000000000000668.
- McMillan Corey T, Irwin David J, Avants BB, Powers J, Cook PA, Toledo JB, McCarty Wood E, Van Deerlin Vivianna M, Lee Virginia M Y, Trojanowski John Q, Grossman Murray. White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2013 Sep;84(9):949-55. doi: 10.1136/jnnp-2012-304418.
- NIH/NINDS R01 NS109260 (current)
- NIH/NIA P01-AG066597 (current, Irwin - Clinical Core Leader, Project Leader)
- NIH/NINDS K23 NS088341 (07/01/14-06/30/19)
- Brightfocus Foundation (07/01/2016 - 06/30/2019)
- Penn IOA (7/1/2016-6/30/2017)


