Lewy body disorders
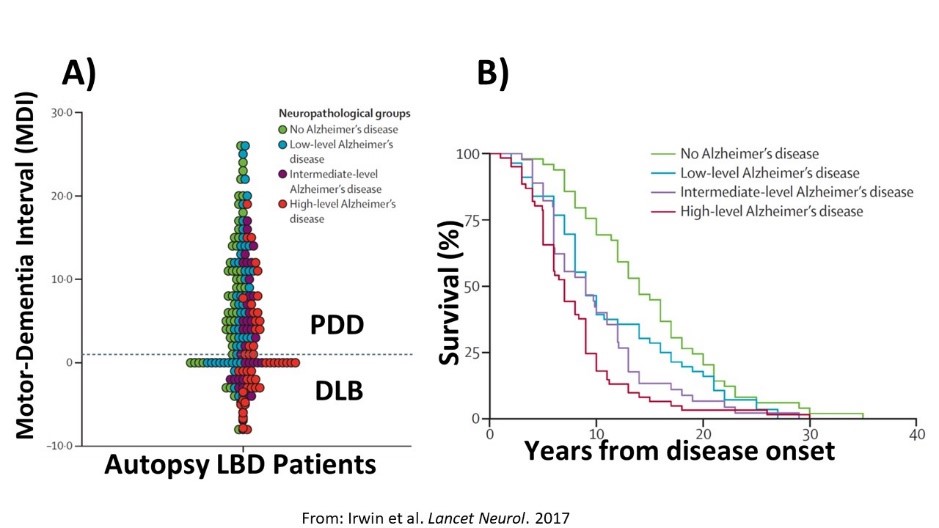
Lewy body disorders (LBD) include Parkinson’s disease (PD), PD with dementia (PDD) and Dementia with Lewy bodies (DLB). These clinical syndromes are associated with underlying alpha-synuclein proteinopathy (i.e. Lewy bodies and Lewy neurites composed of misfolded alpha-synculein protein) at autopsy (Figure 1); however, there is considerable overlap between PD/PDD and DLB and heterogeneity within each LBD clinical diagnosis which is not clearly understood. This raises a significant problem for clinical care as prognostic markers are lacking. Indeed, the majority of PD will develop cognitive impairment and dementia during the course of illness but there is a large variability in the timing of dementia, with some patients developing dementia early in the course of disease compared to those PD patients who have motor symptoms for a decade or more before developing cognitive impairment. Moreover, the classic features of dementia in DLB (i.e. well-formed visual hallucinations, fluctuations in attention) and also non-cognitive symptoms of DLB (i.e. motor Parkinsonism, autonomic dysfunction, rapid-eye-movement sleep behavior disorder- REM SBD and neuroleptic sensitivity) are common in PDD. Indeed, current clinical criteria for PDD and DLB are largely overlapping with the exception of the “one-year rule” that differentiates dementia in the setting of established PD (i.e. PDD with dementia > one year after the onset of PD) from DLB where dementia may precede or occur within one year of the onset of motor Parkinsonism. Thus, there is a clinicopathological spectrum of LBD with varying cognitive, motor and autonomic symptoms that emerge in disease. Previous work by our group and others find that additional Alzheimer’s disease (AD) co-neuropathology (amyloid-beta plaques and tau neurofibrillary tangles) are common in LBD (~50% of all LBD have sufficient AD neuropathologic change sufficient for a secondary neuropathological diagnosis of medium/high AD= SYN+AD), is associated with greater alpha-synuclein pathology in brain (Figure 1) and has a strong influence on clinical features in both PD/PDD and DLB, including earlier dementia and worse survival that is independent of age (Figure 2).
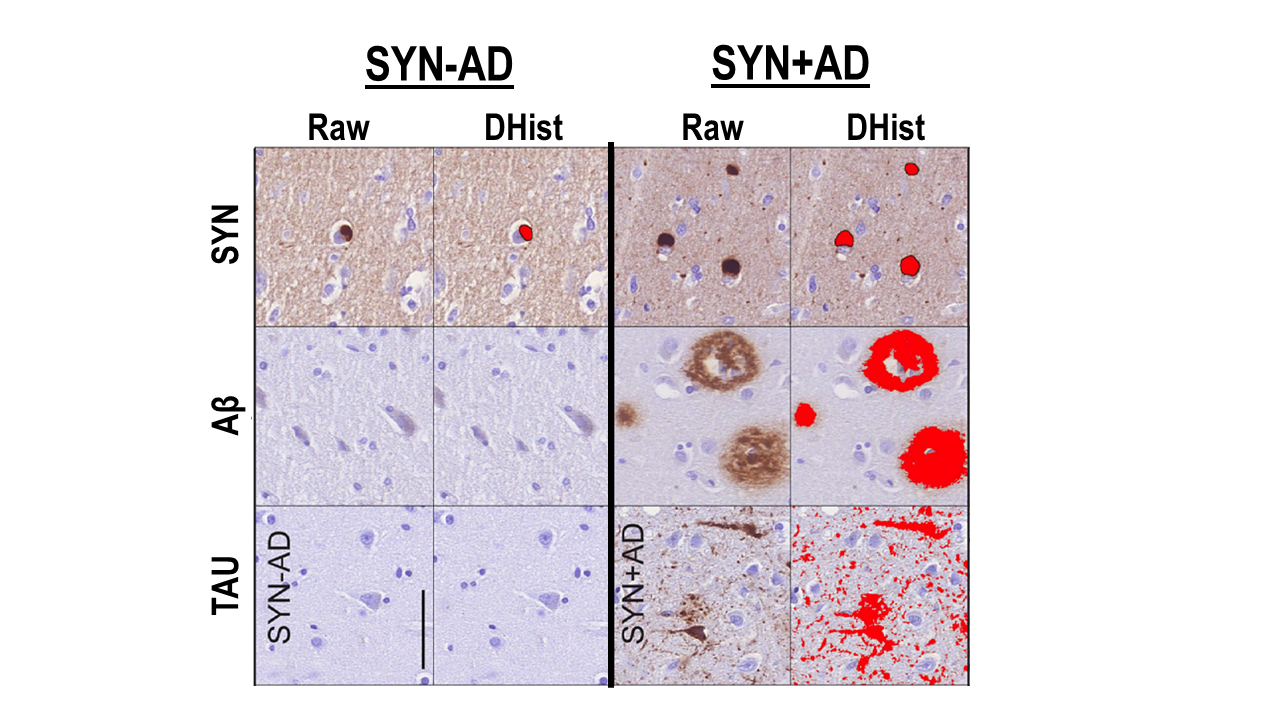
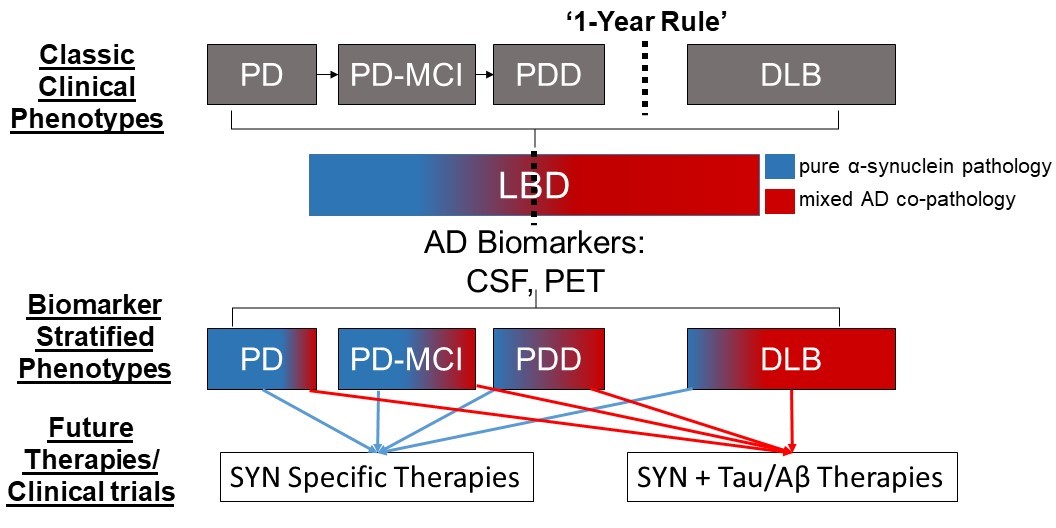
We hypothesize that AD co-pathology is intimately involved in the neurodegenerative process of LBD, and based on animal and cell-model data, we believe there is a synergistic interaction of alpha-synuclein and tau pathology. Our recent work with digital histology finds that tau pathology has a strong influence on clinical features of dementia in LBD and is associated with the regional distribution of alpha-synculein that diverges from the classic patterns of tau pathology in the brain associated with AD without alpha-synuclein co-pathology. Moreover, emerging work in living patients using biomarkers for AD neuropathology (e.g. CSF, PET molecular imaging) in clinical and autopsy-confirmed LBD patients find important prognostic implications and reflect the deposition of these pathologies in vivo. Thus, we propose natural history studies and clinical trials for LBD should consider AD biomarker profiles to stratify LBD into more biologically meaningful groups (i.e. LBD with AD co-pathology vs LBD without AD co-pathology) than classic LBD clinical syndromes alone (i.e. PD, PDD and DLB) (Figure 3). Please see our publication list below for recent work on this topic.
- Tau pathology associates with in vivo cortical thinning in Lewy body disorders.
Annals of clinical and translational neurology. 2020 Dec;7(12):2342-2355 Epub 2020 Oct 27; Authors: Spotorno N, Coughlin DG, Olm CA, Wolk D, Vaishnavi SN, Shaw LM, Dahodwala N, Morley JF, Duda JE, Deik AF, Spindler MA, Chen-Plotkin A, Lee EB, Trojanowski JQ, McMillan CT, Weintraub D, Grossman M, Irwin DJ - Hippocampal subfield pathologic burden in Lewy body diseases vs. Alzheimer's disease.
Neuropathology and applied neurobiology. 2020 Dec;46(7):707-721 Epub 2020 Sep 24; Authors: Coughlin DG, Ittyerah R, Peterson C, Phillips JS, Miller S, Rascovsky K, Weintraub D, Siderowf AD, Duda JE, Hurtig HI, Wolk DA, McMillan CT, Yushkevich PA, Grossman M, Lee EB, Trojanowski JQ, Irwin DJ - Multimodal in vivo and postmortem assessments of tau in Lewy body disorders.
Neurobiology of aging. 2020 Dec;96:137-147 Epub 2020 Aug 21; Authors: Coughlin DG, Phillips JS, Roll E, Peterson C, Lobrovich R, Rascovsky K, Ungrady M, Wolk DA, Das S, Weintraub D, Lee EB, Trojanowski JQ, Shaw LM, Vaishnavi S, Siderowf A, Nasrallah IM, Irwin DJ, McMillan CT, Alzheimer’s Disease Neuroimaging Initiative. - Irwin DJ, Fedler J, Coffey CS, Caspell-Garcia C, Kang JH, Simuni T, Foroud T, Toga AW, Tanner CM, Kieburtz K, Chahine LM, Reimer A, Hutten S, Weintraub D, Mollenhauer B, Galasko DR, Siderowf A, Marek K, Trojanowski JQ, Shaw LM. Evolution of Alzheimer's Disease Cerebrospinal Fluid Biomarkers in Early Parkinson's Disease [published online ahead of print, 2020 Jun 16]. Ann Neurol. 2020;10.1002/ana.25811. doi:10.1002/ana.25811
- Coughlin DG, Hurtig HI, Irwin DJ. Pathological Influences on Clinical Heterogeneity in Lewy Body Diseases. Mov Disord. 0885-3185, Oct 2019. doi:10.1002/mds.27867.
- Coughlin David, Xie Sharon X, Liang Mendy, Williams Andrew, Peterson Claire, Weintraub Daniel, McMillan Corey T, Wolk David A, Akhtar Rizwan S, Hurtig Howard I, Branch Coslett H, Hamilton Roy H, Siderowf Andrew D, Duda John E, Rascovsky Katya, Lee Edward B, Lee Virginia M-Y, Grossman Murray, Trojanowski John Q, Irwin David J*: Cognitive and Pathological Influences of Tau Pathology in Lewy Body Disorders. Annals of neurology 85(2): 259-271, Feb 2019. doi: 10.1002/ana.25392.
- Irwin David J, Xie Sharon X, Coughlin David, Nevler Naomi, Akhtar Rizwan S, McMillan Corey T, Lee Edward B, Wolk David A, Weintraub Daniel, Chen-Plotkin Alice, Duda John E, Spindler Meredith, Siderowf Andrew, Hurtig Howard I, Shaw Leslie M, Grossman Murray, Trojanowski John Q: CSF tau and amyloid-beta predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 90(12): e1038-e1046, Mar 2018. doi: https://doi.org/10.1212/WNL.0000000000005166.
- Irwin David J, Grossman Murray, Weintraub Daniel, Hurtig Howard I, Duda John E, Xie Sharon X, Lee Edward B, Van Deerlin Vivianna M, Lopez Oscar L, Kofler Julia K, Nelson Peter T, Jicha Gregory A, Woltjer Randy, Quinn Joseph F, Kaye Jeffery, Leverenz James B, Tsuang Debby, Longfellow Katelan, Yearout Dora, Kukull Walter, Keene C Dirk, Montine Thomas J, Zabetian Cyrus P, Trojanowski John Q: Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. The Lancet. Neurology 16(1): 55-65, Jan 2017. https://doi.org/10.1016/S1474-4422(16)30291-5.
- Irwin David J, White Matthew T, Toledo Jon B, Xie Sharon X, Robinson John L, Van Deerlin Vivianna, Lee Virginia M-Y, Leverenz James B, Montine Thomas J, Duda John E, Hurtig Howard I, Trojanowski John Q: Neuropathologic substrates of Parkinson disease dementia. Annals of neurology 72(4): 587-98, Oct 2012. doi: 10.1002/ana.23659.
- LBDA Research Center of Excellence (current)
- LBDA Research Center of Excellence (current, Irwin - co-PI)
- NIH/NINDS U01-NS100610 (current, Irwin= co-I)
- NIH/NIA U19-AG062418 (current, Irwin=co-I)


