The IMPRES Study
Did you know?
Undergoing surgically-induced menopause could put you at increased risk for issues with concentration, memory and attention.
Dr. C. Neill Epperson and her research team at the Penn Center for Women's Behavioral Wellness are seeking women to help study the effects of a stimulant medication called Vyvanse ® on memory and attention in women who underwent surgically-induced menopause via risk-reducing salpingo-oophorectomy (RRSO).
You may qualify for study participation if:
- You are between the ages of 35 and 58
- You underwent surgically induced menopause within the last 15 years
- You were premenopausal at the time of surgery
- You are experiencing cognitive difficulties
- You are otherwise medically healthy
*Please note that women who have previously had cancer (breast, ovarian, uterine, other) may also qualify.
To see if you might qualify for this study:
complete a pre-screening questionnaire.
 Dr. Epperson, who holds dual appointments in the Departments of Psychiatry at Penn Medicine and Colorado University School of Medicine, is internationally renowned for her research in women's behavioral health. Dr. Epperson was one of 30 University of Pennsylvania physicians recognized as a "2011- 2012 Best Doctors in America," selected by a consensus of her peers. The quality of Dr. Epperson's research has been recognized as outstanding by her peers as evidenced by her extensive grant funding and research publications in top scientific journals. Dr. Epperson has more than 2 decades of experience in the evaluation and treatment of women with behavioral health issues occurring during periods of hormonal change.
Dr. Epperson, who holds dual appointments in the Departments of Psychiatry at Penn Medicine and Colorado University School of Medicine, is internationally renowned for her research in women's behavioral health. Dr. Epperson was one of 30 University of Pennsylvania physicians recognized as a "2011- 2012 Best Doctors in America," selected by a consensus of her peers. The quality of Dr. Epperson's research has been recognized as outstanding by her peers as evidenced by her extensive grant funding and research publications in top scientific journals. Dr. Epperson has more than 2 decades of experience in the evaluation and treatment of women with behavioral health issues occurring during periods of hormonal change.
Does my doctor need to give me permission to participate in this study?
You do not need your doctor's permission to participate in this study. In order to qualify for this study, you will need to receive a physical examination and EKG. The physical examination includes a discussion of your medical and surgical history, any medications you may be currently taking, a brief assessment of your neurological system, heart, and lungs as well as an EKG and bloodwork. You may complete the physical exam and EKG with our study personnel, or with your own medical doctor. You may speak with your doctor about your participation in this clinical trial, if you care to do so.
What are the risks to participating in this study?
There are potential risks to taking study medication, and completing some of the
procedures involved in this research study (i.e. blood drawing and brain imaging). The research team and the study physician or nurse will discuss risks and benefits of the research study and ingesting study medication when you come in for a study visit.
Will I have to pay for anything if I participate in this study?
You will not be expected to pay for anything for this study.
I do not live in the greater Philadelphia area. Can I still participate in this study?
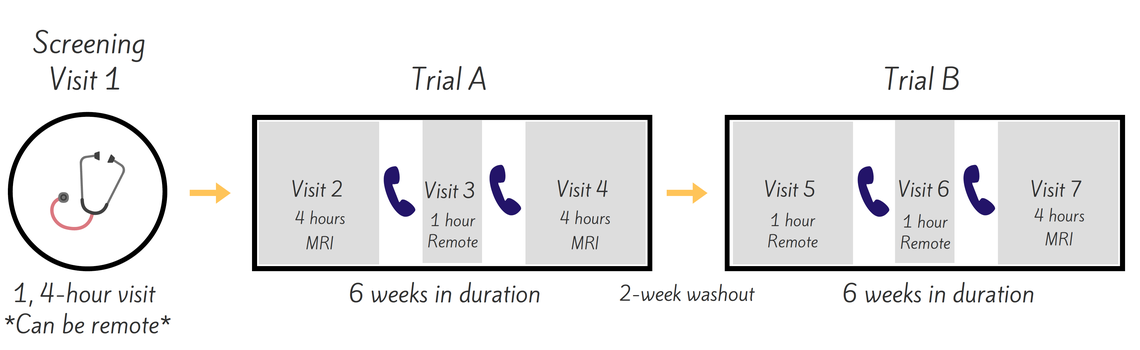
The IMPRES study necessitates 3 in-person study visits to our facilities at the University of Pennsylvania School of Medicine. The 4 remaining visits can be completed remotely if you choose. Reimbursement is available for mandatory in-person visits for this trial.
Who is sponsoring this study?
The National Cancer Institute is the primary sponsor for this study
Epperson CN, Shanmugan S, Kim DR, et al. New onset executive function difficulties at menopause: a possible role for lisdexamfetamine. Psychopharmacology (Berl). 2015;232(16):3091-100.
Shanmugan S, Loughead J, Nanga RP, Elliott M, Hariharan H, Appleby D, Kim D, Ruparel K, Reddy R, Brown TE, Epperson CN. Lisdexamfetamine Effects on Executive Activation and Neurochemistry in Menopausal Women with Executive Function Difficulties. Neuropsychopharmacology. 2017 Jan;42(2):437-445. doi: 10.1038/npp.2016.162. Epub 2016 Aug 23.